Enhancer of anti-tumor drug and application of enhancer
An anti-tumor drug and enhancer technology, applied in the field of biomedicine, can solve problems such as unsatisfactory efficacy, unbearable patient suffering, heterogeneous reactions, etc., and achieve the effects of wide application, improved selectivity, and reduced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
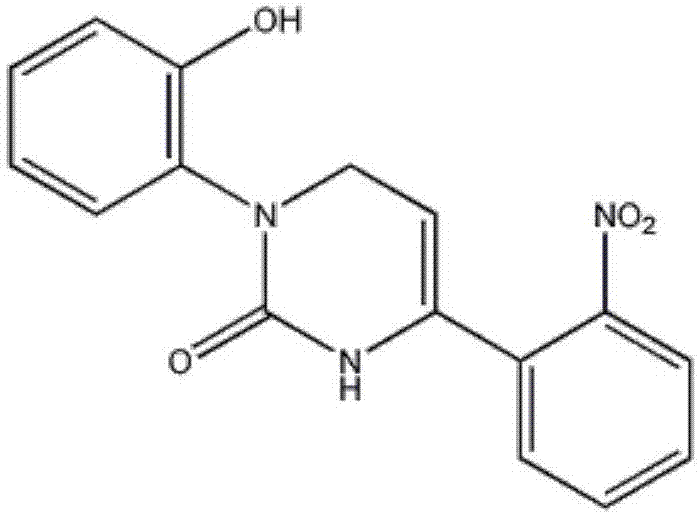
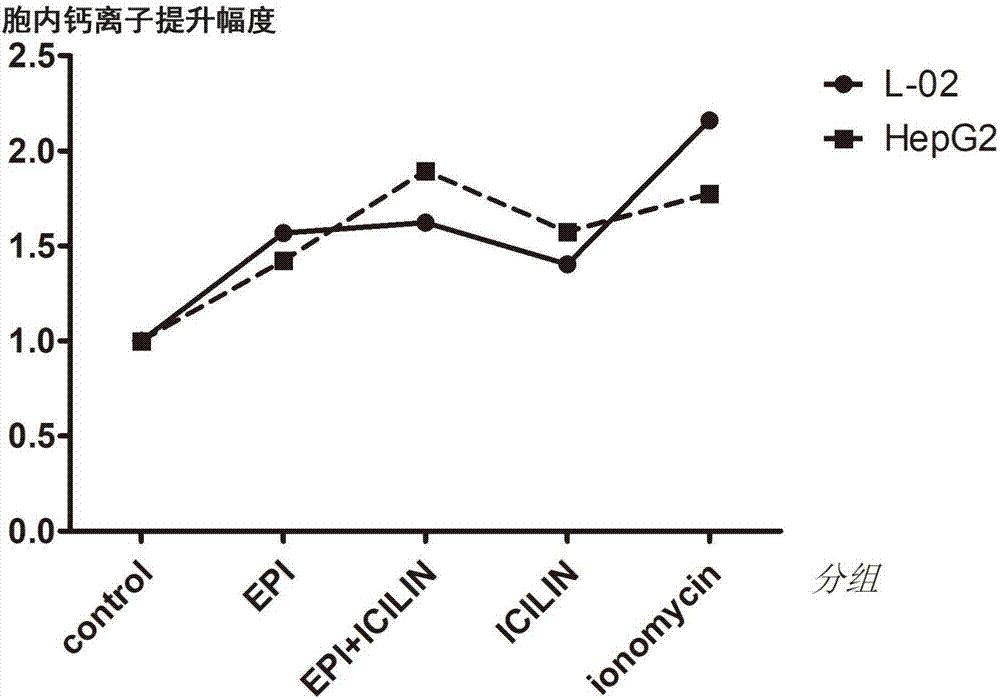
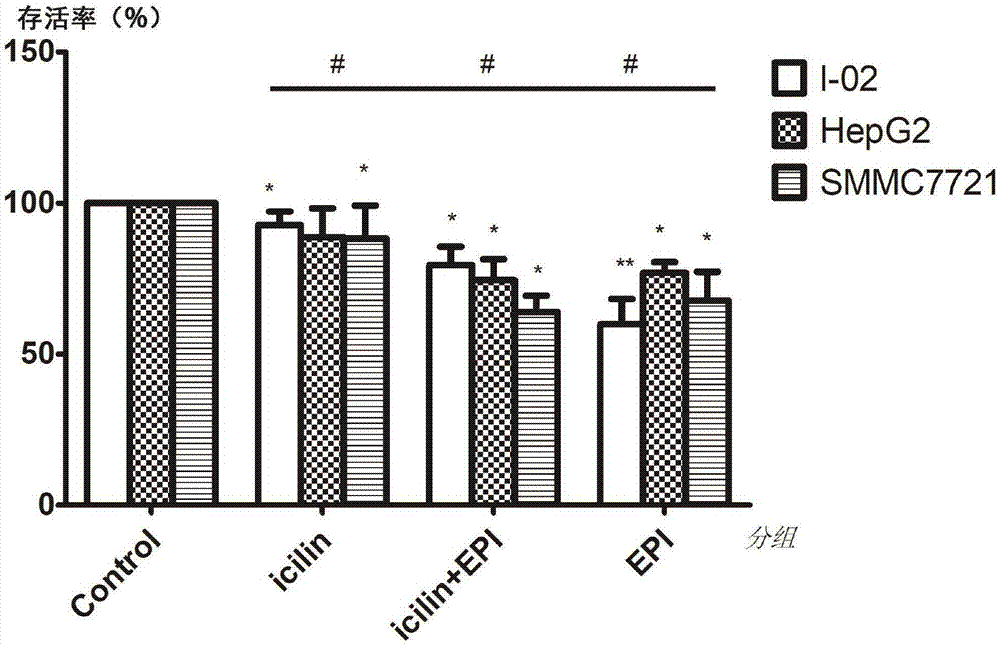
[0031] Such as figure 2 , image 3 , Figure 5 As shown, the present embodiment 1 is to use the group without any treatment as a control group, 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydro Under the condition that the final concentration of pyrimidin-2-one is 75 μM, the changes of cell survival rate, intracellular calcium ion and total intracellular reactive oxygen species in different administration regimens. The preparation method of 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydropyrimidin-2-one is carefully packaged on a 4°C temperature control box Open it, add 1070.81 μL of anhydrous DMSO to the tube with a total mass of 10 mg to dissolve, and prepare a 30 mmol / L stock solution. Before adding the drug, dilute to 75 μM with DMEM culture solution containing 5% fetal bovine serum as needed to ensure DMSO does not exceed 0.5% by volume. The concentration of epirubicin in each culture system was 0.1 μM.
[0032] In terms of cell treatment, the cells i...
Embodiment 2
[0037] Such as Figure 4 As shown, in Example 2, the group without any treatment was used as the control group, and 600 μM of 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6 - Adding tetrahydropyrimidin-2-one into the culture system, the cell treatment, detection method, grouping and legend are the same as in Example 1. The survival rate of the two cell lines was detected by SRB method. Figure 4 In , we found that adding 600 μM 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydropyrimidin-2-one alone had an effect on three kinds of cells HepG2, The inhibition rates of SMMC7721 and L-02 were significantly increased. Although the survival rate of L-02 is still higher than that of the other two liver cancer cell lines, its average survival rate has dropped below 50%, even lower than that of epirubicin alone, which shows that 600 μM 1-(2- Hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydropyrimidin-2-one had a certain inhibitory effect on the proliferation of the three cell ...
Embodiment 3
[0039] Such as Figure 6 As shown, the cell treatment method of this Example 3 is the same as that of Examples 1 and 2, and the group without any treatment is used as a control group, and 300 μM of 1-(2-hydroxyphenyl)-4-(3-nitro Phenyl)-1,2,3,6-tetrahydropyrimidin-2-one was added to the culture system, and the survival rate of the two cell lines was detected by the SRB method. Such as Figure 6 As shown, in the case of 300 μM 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydropyrimidin-2-one alone, the drug The inhibitory effect on HepG2 cell proliferation was significantly higher than that on L02 cell proliferation (p<0.05). In the combined group, with the addition of 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydropyrimidin-2-one, human embryonic liver Although the survival rate of cell L-02 was higher than that of 1-(2-hydroxyphenyl)-4-(3-nitrophenyl)-1,2,3,6-tetrahydropyrimidin-2-one alone decreased, but the survival rate was still higher than 50%, and its...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap