Multidrug infusion for pain control
A technology of preparations and antagonists, which is applied in the field of multi-drug injection for pain control, and can solve problems such as poor control of pain and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0156] Pain Management via Intrathecal Infusion
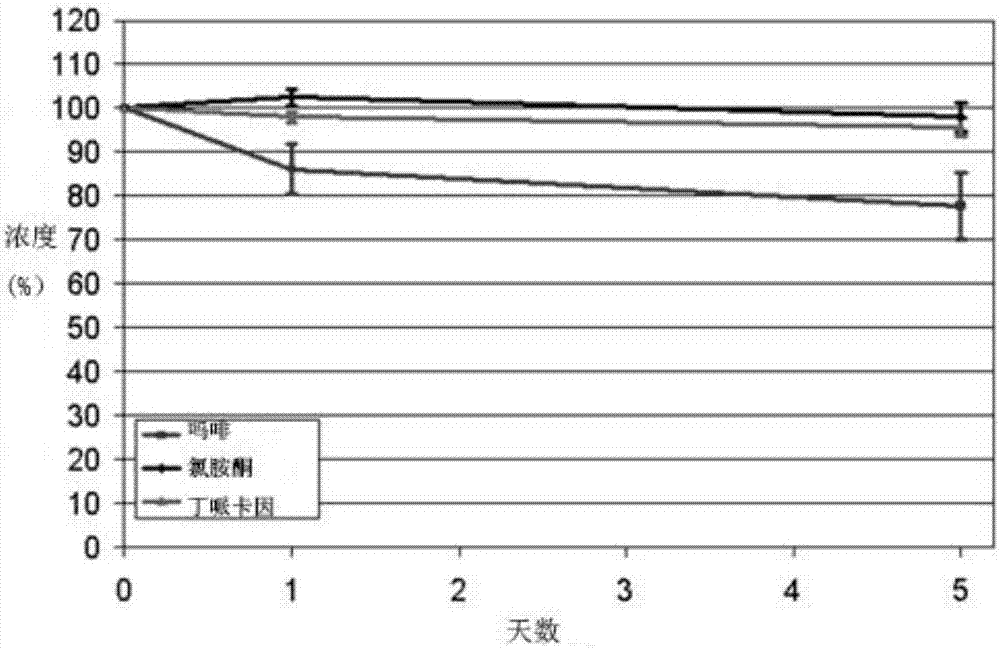
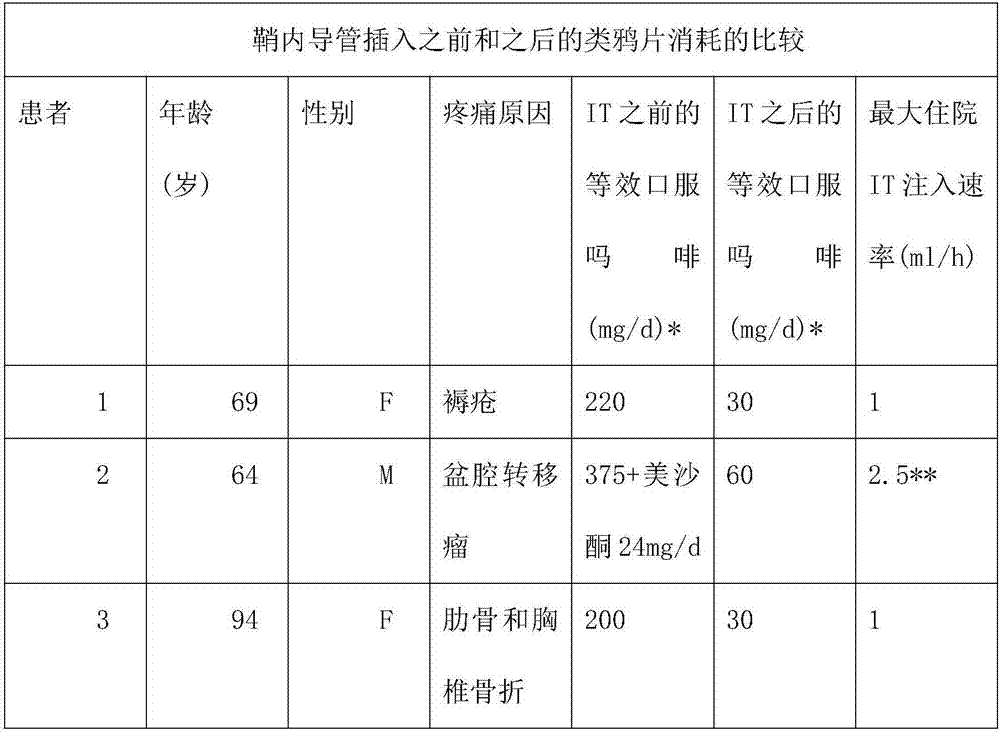
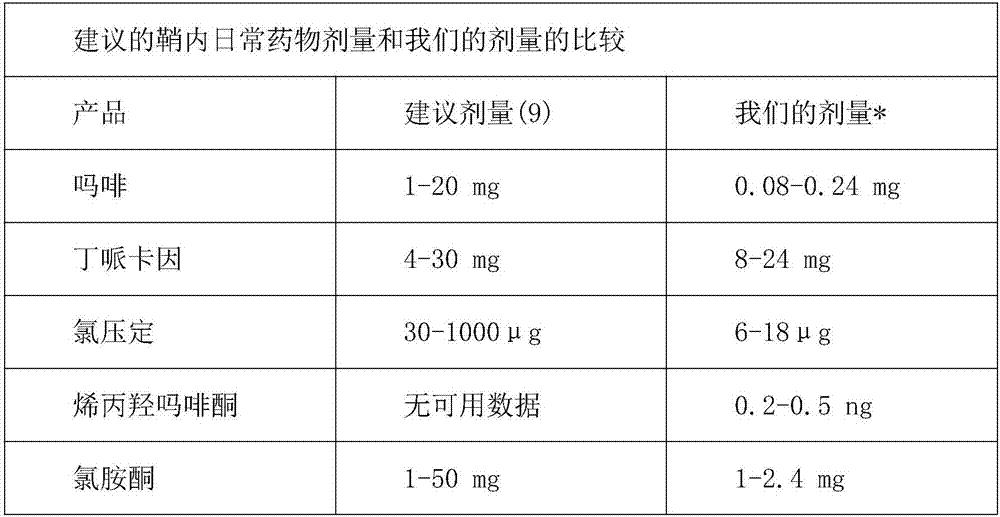
[0157] To explore the efficacy of alternative methods of pain management based on time-limited intrathecal (IT) infusions of anesthetic agent mixtures, patients with refractory and poorly controlled pain due to decubitus ulcers, pelvic metastatic masses, and thoracic spine and rib fractures were treated, respectively. The three patients in the study were 69, 64 and 94 years old. Due to side effects, daily doses of opioids should not be increased. Intrathecal catheter 20G is placed percutaneously in the lumbar region while being advanced thoracically into the channel and secured subcutaneously. It was connected to an external infusion pump with a mixture of bupivacaine 1 mg / ml, oxymorphone 0.02 ng / ml, ketamine 100 μg / ml, morphine 0.01 mg / ml and clonidine 0.75 μg / ml. The initial rate was 1 ml / h. Pain is mainly controlled at a rate of less than 1ml / h. Opioid consumption has been greatly reduced. The catheter was maintained fo...
example 2
[0190] Pain management via infusion in the right brachial plexus
[0191] A 66-year-old elderly patient with subarachnoid hemorrhage (SAHI grade) with ruptured aneurysm of the carotid artery left middle cerebral artery vasospasm. The patient presents with severe right hemiplegic spasticity following a stroke. The spasticity caused significant pain due to contractures and poor positioning of the right arm. It has dysesthesia, allodynia, and hyperalgesia to touching surfaces. The patient also had problems with aphasia, but communicated well.
[0192] Conventional treatments for pain and drug trials (Botox in 2011 and Baclofen 10 mg base in 2013) yielded minimal results and provided no relief.
[0193] The patient had been seeing the pain clinic since February 2014, and intravenous infusions of ketamine were well tolerated but did not provide any relief in pain levels. Blockage of the right arm at the level of the brachial plexus with analgesic solution has been performed wit...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap