Novel self-assembled collagen and its preparation method
A collagen and self-assembly technology, applied in the field of protein engineering, can solve the problems of the preparation method of collagen samples and the complex self-assembly process of collagen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of self-assembled collagen, taking the preparation of self-assembled bovine type I collagen as an example.
[0034] Self-assembled collagen was prepared by using 5 mg / ml bovine type I collagen aqueous solution as raw material.
[0035] (1) The concentration of collagen is 8000 U / g according to E / S. First, trypsin (Trypsin) is added and hydrolyzed at 37°C for 3 hours. During the hydrolysis, the pH is adjusted with 0.1mol / L NaOH to maintain the pH of the reaction system at a constant value of 8.0. Inactivate the enzyme in a boiling water bath for 20 minutes; add alkaline protease (Alcalase) to continue hydrolysis, and hydrolyze at 55°C for 3 hours. cool down. Finally, centrifuge at 5000g / min for 10min, discard the precipitate, and collect the supernatant.
[0036] (2) Ultrafiltration is performed on the hydrolyzate obtained in step (1) with a 1 kDa ultrafiltration membrane to obtain a collagen polypeptide solution, which is placed in a refrigerator at 4° C. ...
Embodiment 2
[0041] Preparation of self-assembled collagen, taking the preparation of self-assembled fish skin type I collagen as an example.
[0042] Self-assembled collagen was prepared by using 5mg / ml fish skin type I collagen aqueous solution as raw material.
[0043] (1) The concentration of collagen is 8000U / g according to E / S. First, add trypsin (Trypsin) and hydrolyze at 37°C for 6 hours. During the hydrolysis, use 0.1mol / L NaOH to adjust the pH to keep the pH of the reaction system at a constant value of 8.0. Inactivate the enzyme in a boiling water bath for 20 minutes; add alkaline protease (Alcalase) to continue hydrolysis, and hydrolyze at 55°C for 6 hours. During the hydrolysis, use 0.1mol / L NaOH to adjust the pH to keep the pH of the reaction system at a constant value of 8.5; cool down. Finally, centrifuge at 5000g / min for 10min, discard the precipitate, and collect the supernatant.
[0044] (2) Ultrafiltration is performed on the hydrolyzate obtained in step (1) with a 3k...
Embodiment 3
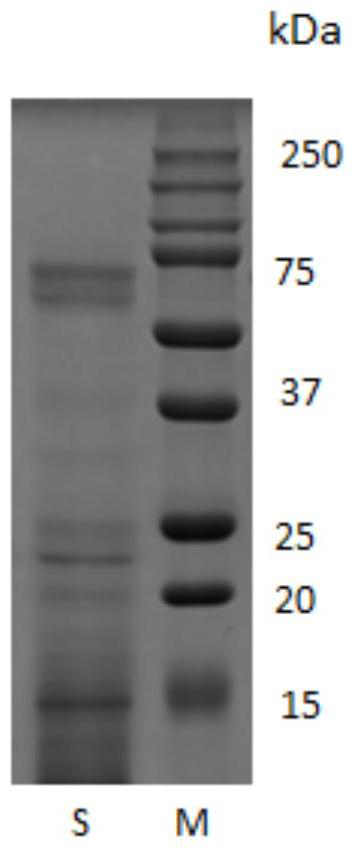
[0047] Example 3 Electrophoresis analysis of the obtained collagen polypeptide and self-assembled collagen polypeptide
[0048] Select the collagen polypeptide solution obtained in Example 1 and the collagen polypeptide solution after self-installation to carry out electrophoresis analysis, specifically as follows:
[0049] SDS-PAGE gel electrophoresis analysis
[0050] The bovine type I collagen polypeptide before and after self-assembly obtained in Example 1 was identified by SDS-PAGE gel electrophoresis at 5% stacking gel concentration, 20% and 12% separating gel concentration.
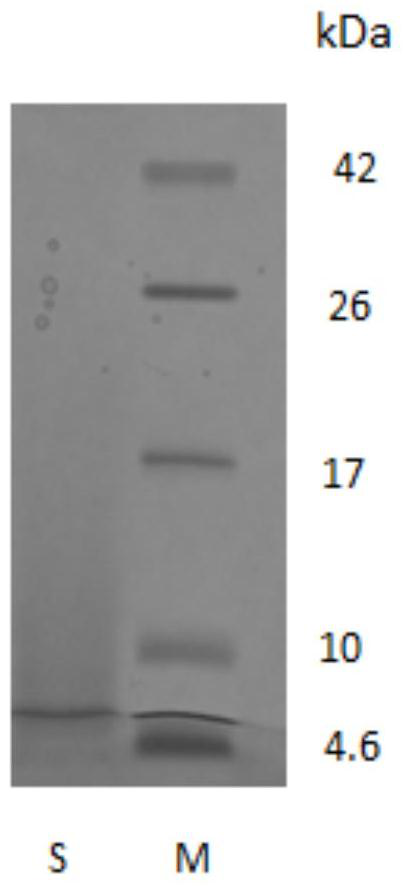
[0051] The result is as Figure 1a As shown, comparing collagen with standard protein Maker, it can be seen that the molecular weight of bovine collagen polypeptide after hydrolysis becomes smaller, and most of them are concentrated at about 6.8kDa. S in the figure represents the bovine type I after protease hydrolysis in Example 1 Collagen peptides.
[0052] The result is as Figure 1b As shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com