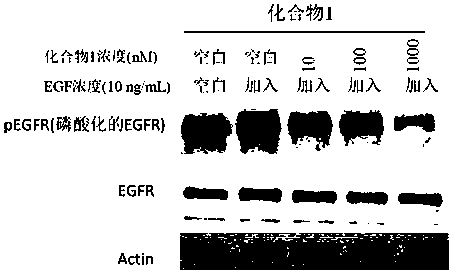
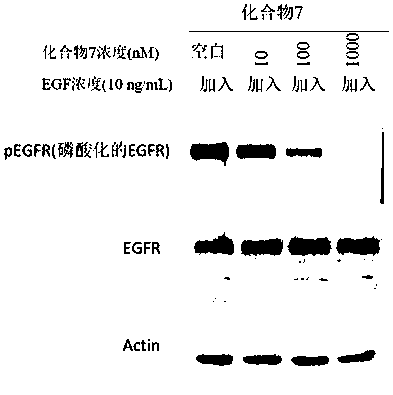
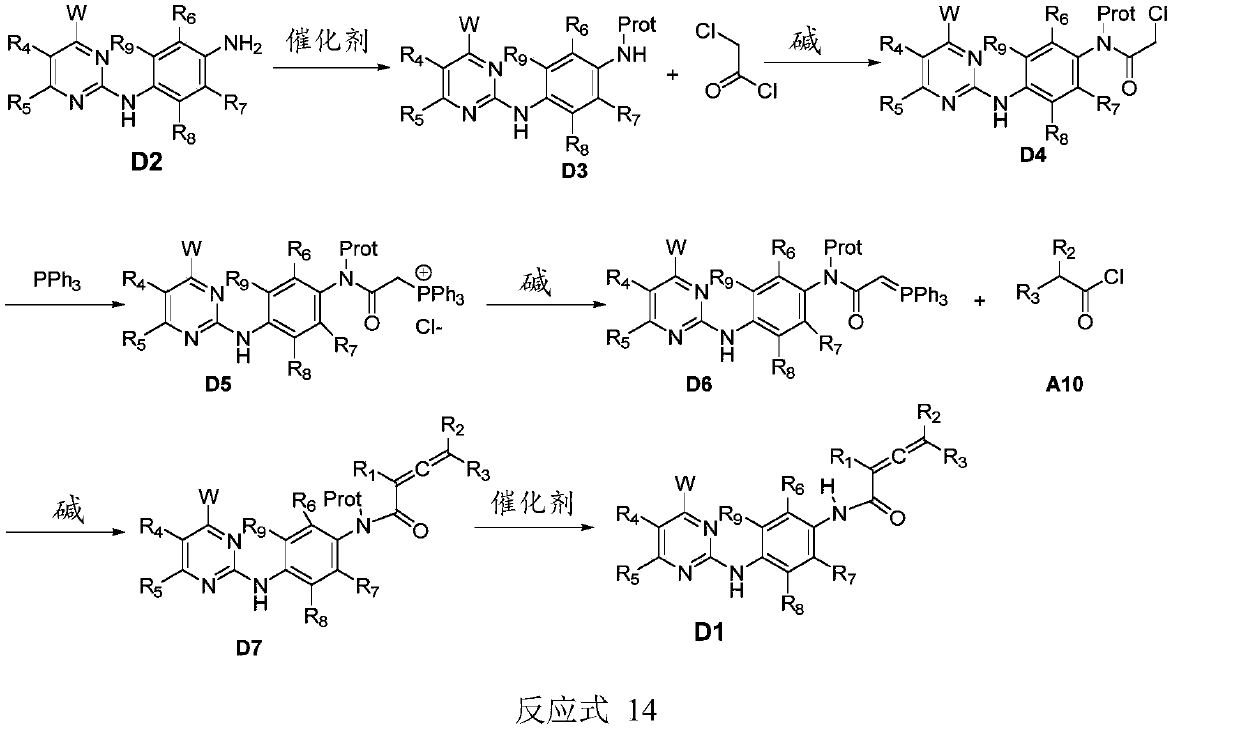
Compound containing conjugated allenamide structure, preparation method, pharmaceutical composition and use thereof
A compound, selected technology, applied in the direction of drug combination, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of compound biological activity, uncertainty in the application prospect of pharmacological properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Example 1 Synthesis of N-[2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1H-ind Indol-3-yl)pyrimidin-2-yl)amino)phenyl]-2,3-butenamide (1)
[0181]
[0182] Step 1: Synthesis of 3-(2-chloropyrimidin-4-yl)-1H-indole (GB011)
[0183] CH 3MgCl (3M in tetrahydrofuran, 14 mL, 42 mmol) was added dropwise to a solution of indole (5 g, 42 mmol) in 1,2-dichloroethane (250 mL). The resulting solution was stirred for 15 minutes, then 2,4-dichloropyrimidine (9.48 g, 64 mmoL) was added in one portion. The resulting solution was allowed to warm to room temperature and stirred for an additional 16 hours. The reaction was quenched by the addition of methanol (25 mL) and the resulting mixture was concentrated in vacuo and the residue was adsorbed onto silica gel and purified using a normal phase silica gel column to afford GB011 (3 g) as a yellowish solid. 1 H NMR (DMSO-d 6 ,400MHz): 7.18-7.27(m,2H),7.46-7.53(m,1H), 7.90(d,1H,J=5.36Hz), 8.38-8.44(m,1H), 8.48...
Embodiment 2
[0198] Example 2 Synthesis of (S)-N-[2-(tetrahydrofuran-3-yl)oxyl-4-methoxyl-5-((4-(1-methyl-1H-indol-3-yl) Pyrimidin-2-yl)amino)phenyl]-2,3-butenamide (2)
[0199]
[0200] Step 1: Synthesis of (S)-N-(2-methoxy-4-(tetrahydrofuran-3-yl)oxy-5-nitro)phenyl-4-(1-methyl-1H-indole- 3-yl)pyrimidin-2-ylamine (GB056)
[0201] Potassium tert-butoxide (0.366g, 3.81mmol) was added into N,N-dimethylformamide (10mL) and stirred at 0°C, and 0.167g (1.9mmol) of (S)-(+)-3-hydroxy Tetrahydrofuran, reacted for 1 hour. Add GB17 (0.500 g, 1.27 mmol) and react at room temperature for 3 hours. Add 100 mL of water to the resulting suspension, adjust the pH to 7-8, stir, filter with suction, dry the filter cake, and purify through a silica gel column to obtain 0.15 g of the target product GB056.1 H NMR (DMSO-d 6 ,400MHz):8.77(s,1H),8.28-8.40(m,3H),8.18(s,1H),7.51(d,1H,J=7.95Hz),7.20-7.28(m,2H),7.07- 7.13(m,1H),6.93(s,1H),5.34-5.41(m,1H),4.00(s,3H),3.93-3.98(m,1H),3.76-3.92(m,2H),3.87( s,3H),...
Embodiment 3
[0206] Example 3 Synthesis of N-[2-(4-methylpiperazinyl)-4-methoxy-5-((4-(1-methyl-1H-indol-3-yl)pyrimidine-2- Base) amino) phenyl] -2,3-butenamide (3)
[0207]
[0208] Step 1: Synthesis of N-(4-(4-methylpiperazinyl)-2-methoxy-5-nitrophenyl)-4-(1-methyl-1H-indol-3-yl) Pyrimidin-2-ylamine (GB066)
[0209] Add GB017 (0.1g, 0.254mmol) to 5mL N-ylpyrrolidone and stir at room temperature, add nitrogen methyl piperazine (0.025g, 0.254mmol), then add diisopropylethylamine (0.1mL), and heat up to Reaction at 140°C. React for 2.5 hours and cool down to room temperature. Add 100mL of water to the above reaction solution, filter with suction, dry the filter cake, and purify through a silica gel column to obtain 0.1g of the target product GB066. 1 H NMR (CDCl 3 ,400MHz):9.66(s,1H),8.40(d,1H,J=5.25Hz),8.29(s,1H),8.14-8.20 (m,1H),7.57(s,1H),7.39-7.44( m,1H),7.28-7.36(m,2H),7.20(d,1H,J=5.29Hz),6.63(s,1H), 4.00(s,3H),3.95(s,3H),3.10-3.19 (m,4H), 2.62-2.72(m,4H), 2.40(s,3H). ESI-MS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com