Anti-ROR1 chimeric antigen receptors
A chimeric antigen receptor, antibody technology, applied in the direction of antibodies, antibody medical components, antibody mimics/scaffolds, etc., can solve the problem of lack of ROR1 expression in adult tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0657] Construction of anti-ROR1 CAR
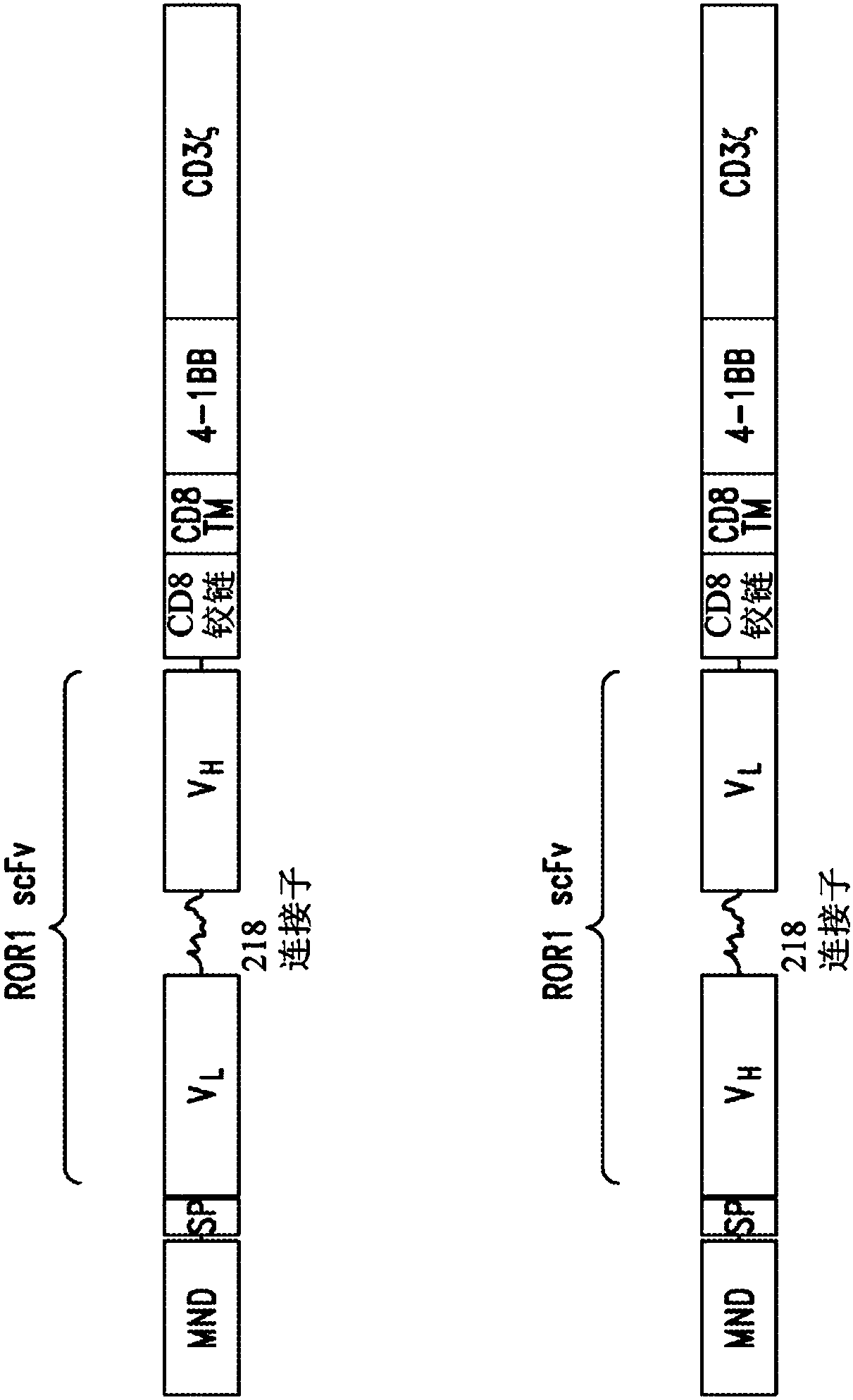
[0658] CARs containing humanized anti-ROR1 scFv antibodies were designed to contain the MND promoter operably linked to anti-ROR1 scFv, hinge and transmembrane domains from CD8α and CD137 costimulatory domains, followed by CD3ζ The intracellular signaling domain of the chain. figure 1 The anti-ROR1 CAR includes a CD8α signal peptide (SP) sequence for surface expression on immune effector cells. Table 3 shows the identity, gene bank reference, source nomenclature, and cited documents of various nucleotide fragments of exemplary anti-ROR1 CAR lentiviral vectors.
[0659] table 3.
[0660]
[0661]
example 2
[0663] Evaluation of Human Anti-ROR1 CAR T Cells
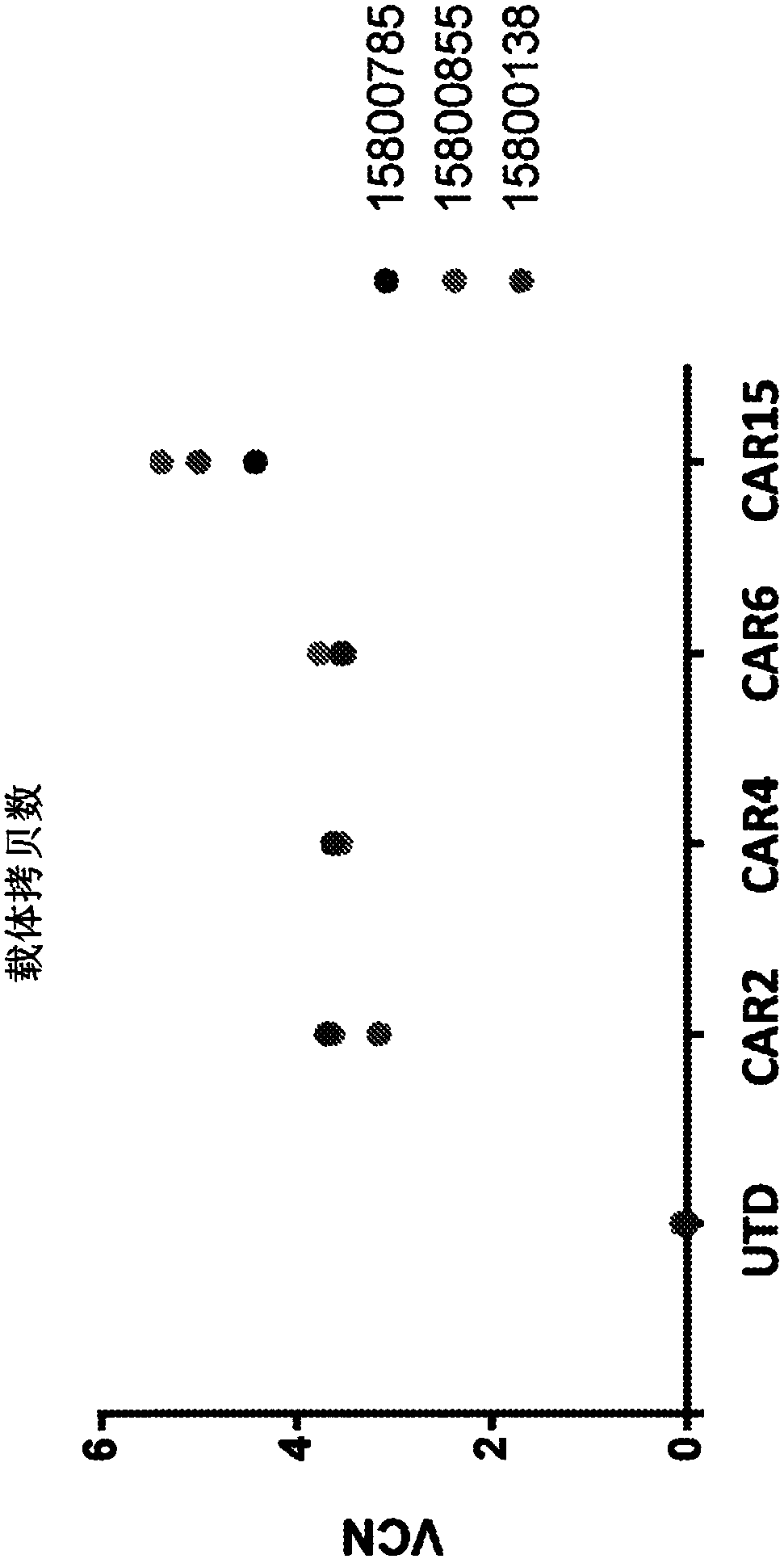
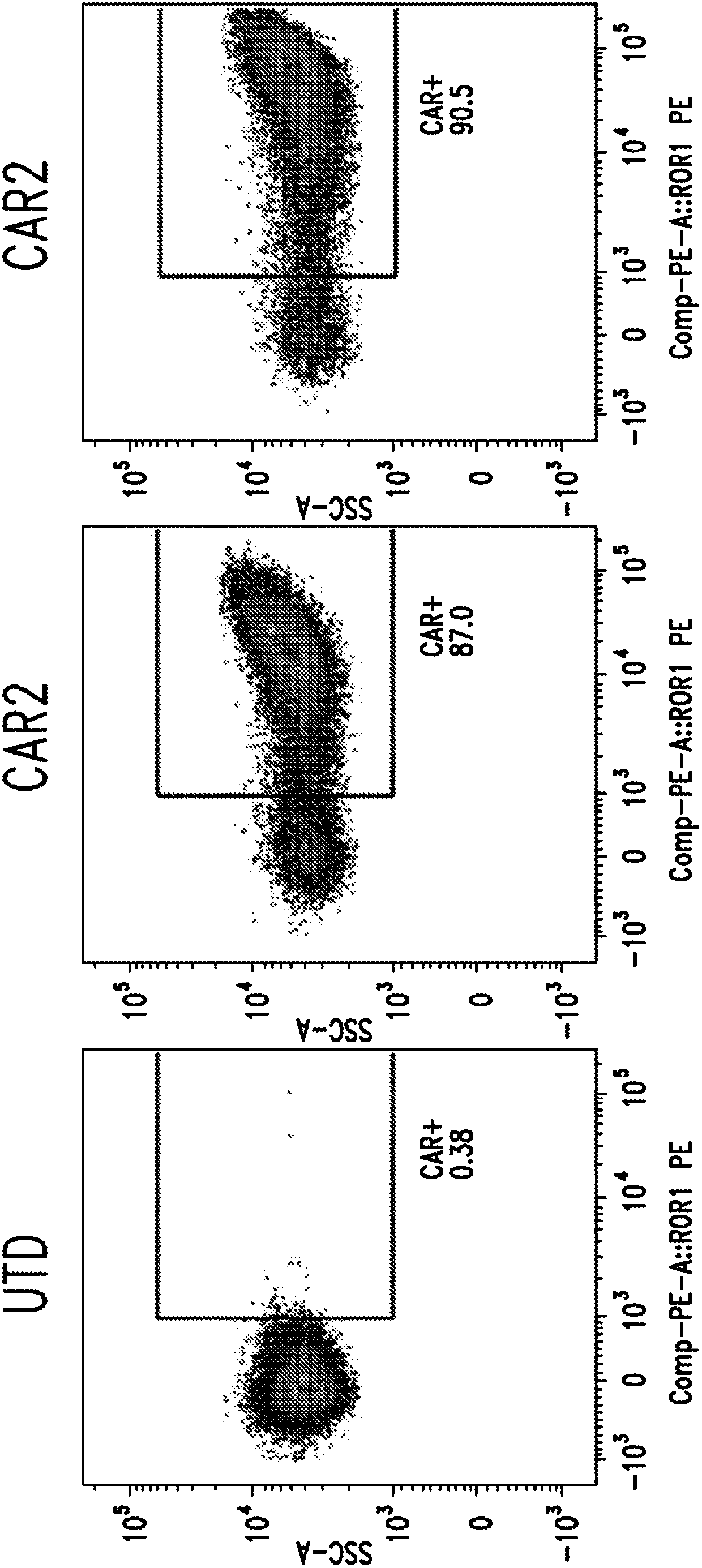
[0664] Evaluation of transduction efficiency, CAR expression, and ROR1 biological activity.
[0665] Anti-ROR1 CAR molecules were constructed using sequences from scFv isolated from a human phage display library. CAR T cells were generated following lentiviral transduction of primary human T cells. Four CAR candidates were selected for further study after an initial high-throughput screen consisting of in vitro assays analyzing CAR expression and ROR1-specific T cell activity. Anti-ROR1 CAR2 includes the variable chain set forth in SEQ ID NO:7 and SEQ ID NO:8; anti-ROR1 CAR4 includes the variable chain set forth in SEQ ID NO:63 and SEQ ID NO:64; anti-ROR1 CAR6 includes The variable chain set forth in SEQ ID NO:15 and SEQ ID NO:16 and the anti-ROR1 CAR15 includes the variable chain set forth in SEQ ID NO:159 and SEQ ID NO:160. The transduction efficiency, CAR expression, and ROR1 biological activity of anti-ROR1 CAR T cells...
example 3
[0672] Evaluation of additional human anti-ROR1 CAR T cells
[0673] Evaluation of transduction efficiency, CAR expression, and ROR1 biological active.
[0674] Anti-ROR1 CAR molecules were constructed using sequences from scFv isolated from a human phage display library. CAR T cells were generated following lentiviral transduction of primary human T cells. Five CAR candidates were selected for further study following an initial high-throughput screen consisting of in vitro assays analyzing CAR expression and ROR1-specific T cell activity. The anti-ROR1 CAR50 includes the variable chain set forth in SEQ ID NO:255 and SEQ ID NO:256; the anti-ROR1 CAR53 includes the variable chain set forth in SEQ ID NO:271 and SEQ ID NO:272; the anti-ROR1 CAR54 includes the variable chain set forth in SEQ ID NO:271 and SEQ ID NO:272; The variable chains in SEQ ID NO:279 and SEQ ID NO:280; the anti-ROR1 CAR60 includes the variable chains set forth in SEQ ID NO:319 and SEQ ID NO:320; and the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com