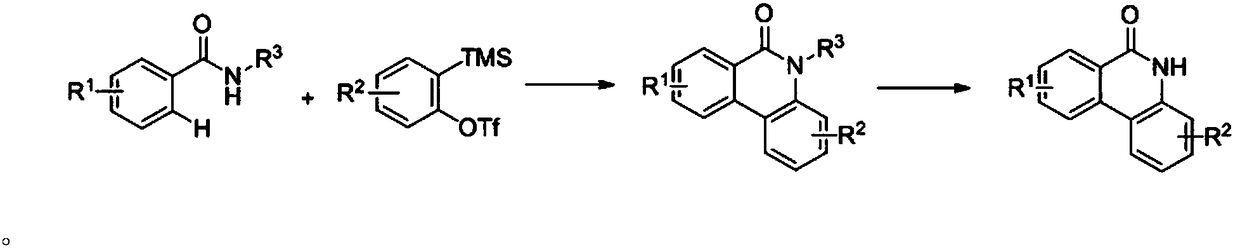
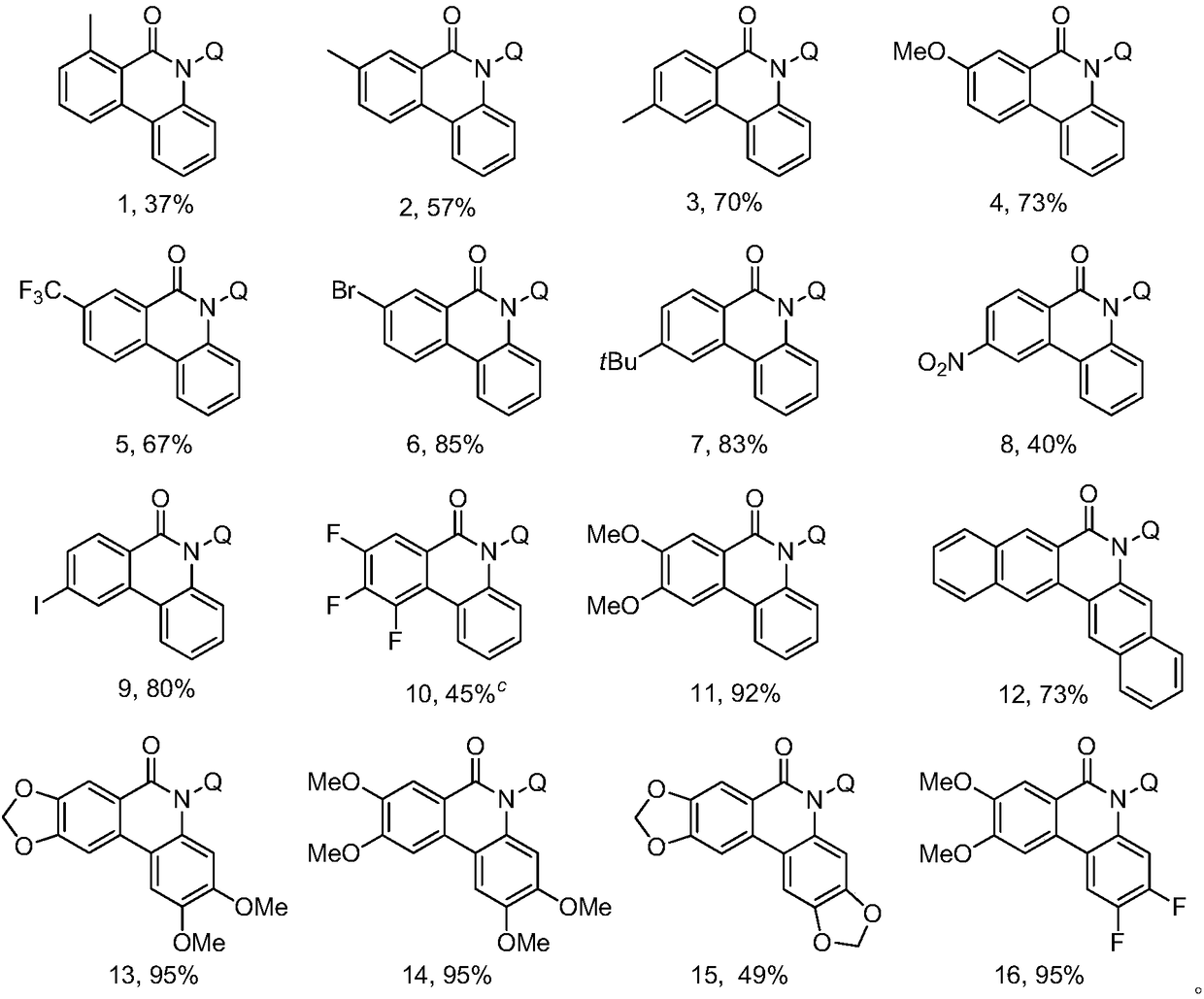
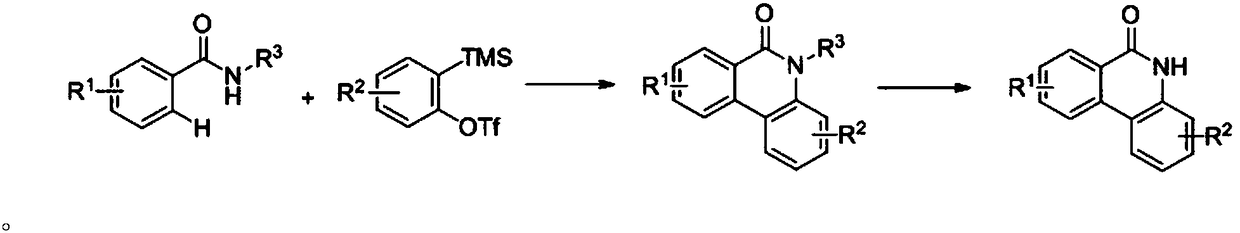
Synthetic method for phenanthridone compound
A synthesis method and compound technology are applied in the field of synthesis of phenanthridine compounds, and can solve the problems of complex synthesis steps of starting materials, limited types of synthesis products, low yield of synthesis routes, etc., and achieve easy operation, mild reaction conditions, Simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of compound intermediate (I-1)
[0042] 0.2mmol 2-methyl-benzoyl-8-quinolinamine, 0.4mmol benzyne precursor, 0.07mmol Cu(OAc) 2 , 0.24mmol cesium fluoride, 0.1mmol tetrabutylammonium iodide, 1mL N,N-dimethylformamide, 1mL MeCN were added to the reaction flask, purged with oxygen, sealed and heated to 80°C for 12h, cooled to room temperature Afterwards, the compound (I-1) was obtained as colorless crystals after distillation and purification under reduced pressure, with a yield of 37%.
[0043] Compound (I-1) is:
[0044] 1 HNMR (CDCl 3,400MHz,ppm):δ8.80(dd,J=4.2,1.7Hz,1H),8.33-8.26(m,3H),8.04-8.00(m,1H),7.77-7.73(m,2H),7.66 (t, J=7.8Hz, 1H), 7.44-7.41(m, 1H), 7.40(d, J=7.4Hz, 1H), 7.21(td, J=7.2, 1.4Hz, 1H), 7.14(td, J=8.5, 1.6Hz, 1H), 6.37(dd, J=8.2, 1.0Hz, 1H), 2.91(s, 3H); 13 CNMR (CDCl 3 ,100MHz,ppm)δ162.97,151.54,144.84,143.34,139.77,136.80,136.49,136.15,132.03,131.89,130.85,130.02,129.33,129.05,127.04,124.61,123.56,122.28,122...
Embodiment 2
[0045] Embodiment 2: the preparation of compound intermediate (I-2)
[0046] 0.2mmol 3-methyl-benzoyl-8-quinolinamine, 0.4mmol benzyne precursor, 0.07mmol Cu(OAc) 2 , 0.24mmol cesium fluoride, 0.1mmol tetrabutylammonium iodide, 1mL N,N-dimethylformamide, 1mL MeCN were added to the reaction flask, purged with oxygen, sealed and heated to 80°C for 12h, cooled to room temperature Afterwards, the compound (I-2) was obtained as colorless crystals after distillation and purification under reduced pressure, with a yield of 57%.
[0047] Compound (I-2) is:
[0048] 1 HNMR (CDCl 3 ,400MHz,ppm):δ8.78(dd,J=4.2,1.7Hz,1H),8.37(s,1H),8.31-8.25(m,3H),8.02(dd,J=7.0,2.7Hz,1H ),7.77-7.72(m,2H),7.63(dd,J=8.3,1.7Hz,1H),7.42-7.39(m,1H),7.23(td,J=8.4,2.3Hz,1H),7.15( td,J=8.4,1.5Hz,1H),6.47(dd,J=8.3,1.0Hz,1H),2.52(s,3H); 13 CNMR (CDCl 3 ,100MHz,ppm)δ162.20,151.50,144.76,139.36,138.22,136.43,134.21,132.11,130.72,129.94,129.47,129.00,128.66,126.90,126.02,122.96,122.52,122.03,119.41,116.95,21....
Embodiment 3
[0049] Embodiment 3: the preparation of compound intermediate (I-3)
[0050] 0.2mmol 4-methyl-benzoyl-8-quinolinamine, 0.4mmol benzyne precursor, 0.07mmol Cu(OAc) 2 , 0.24mmol cesium fluoride, 0.1mmol tetrabutylammonium iodide, 1mL N,N-dimethylformamide, 1mL MeCN were added to the reaction flask, purged with oxygen, sealed and heated to 80°C for 12h, cooled to room temperature Afterwards, the compound (I-3) was obtained as colorless crystals after distillation and purification under reduced pressure, with a yield of 70%.
[0051] Compound (I-3) is:
[0052] 1 HNMR (CDCl 3 ,400MHz,ppm):δ8.78(dd,J=4.2,1.7,1H),8.46(d,J=8.1Hz,1H),8.33(dd,J=7.9,1.4Hz,1H),8.26(dd ,J=8.3,1.7Hz,1H),8.17(s,1H),8.02(dd,J=7.2,2.5Hz,1H),7.78-7.72(m,2H),7.44-7.40(m,2H), 7.25-7.21(m,1H),7.19-7.15(m,1H),6.47(dd,J=8.2,1.0Hz,1H),2.60(s,3H); 13 CNMR (CDCl 3 ,100MHz,ppm)δ162.18,151.54,144.84,143.36,139.88,136.42,134.58,130.78,129.94,129.51,129.46,129.29,129.02,126.91,123.95,123.15,122.45,122.07,122.03,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com