Anti-IL-4R antibody and application thereof
An IL4, antibody technology, applied in the direction of antibody, application, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1. Construction of Phage Display Antibody Library
[0138] The content of this example refers to the inventor’s previous Chinese patent applications No. 201610609651.X (titled: Anti-human PDL1 antibody and its use) and No. 201510097117.0 (titled: Anti-human IL-17 monoclonal antibody). By way of reference, the contents of the above two patent applications are incorporated herein.
Embodiment 2
[0139] Embodiment 2: Preparation of recombinant protein
[0140] A variety of different recombinant proteins were used in the preparation and testing of anti-IL-4R mAbs, including human IL-4R extracellular domain (hIL-4R, SEQ ID NO:1), mouse IL-4R extracellular domain ( mIL-4R, SEQ ID NO:2), macaque IL-4R extracellular region (mmIL-4R, SEQ ID NO:3), human IL-4 extracellular region (hIL-4, SEQ ID NO:4) and human Recombinant IL-13 (SEQ ID NO:32). These proteins all have post-translational modifications (such as: glycosylation or disulfide bonds, etc.), so the use of mammalian cell expression systems will be more conducive to maintaining the structure and function of recombinant proteins. In addition, a His tag (His, SEQ ID NO: 5) or the Fc fragment of human antibody IgG1 (Fc, SEQ ID NO: 6) or the Fc fragment of mouse IgG2a (mFc, SEQ ID NO: 6) was added to the C-terminus of these recombinant proteins. NO:7), which is more conducive to the purification of recombinant proteins an...
Embodiment 3
[0143] Example 3: Screening of anti-human IL4R monoclonal antibody using phage display antibody library technology
[0144] 3.1 Screening of anti-human IL4R monoclonal antibody
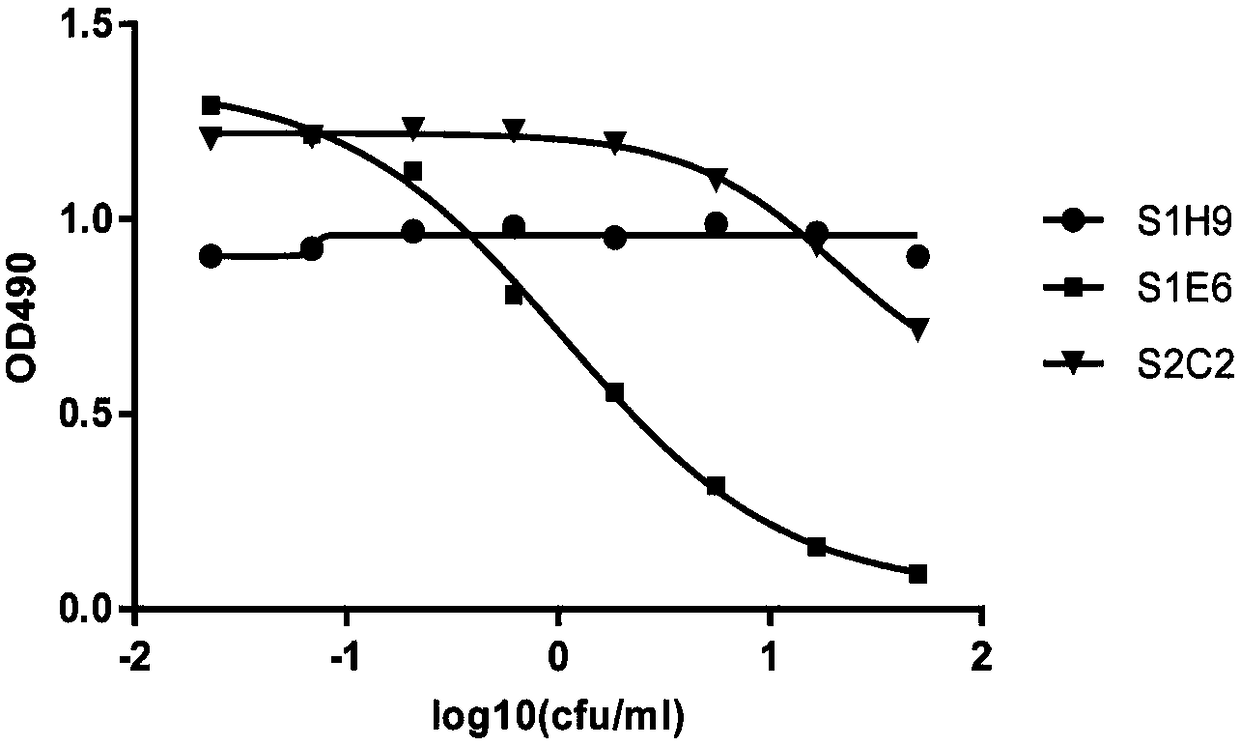
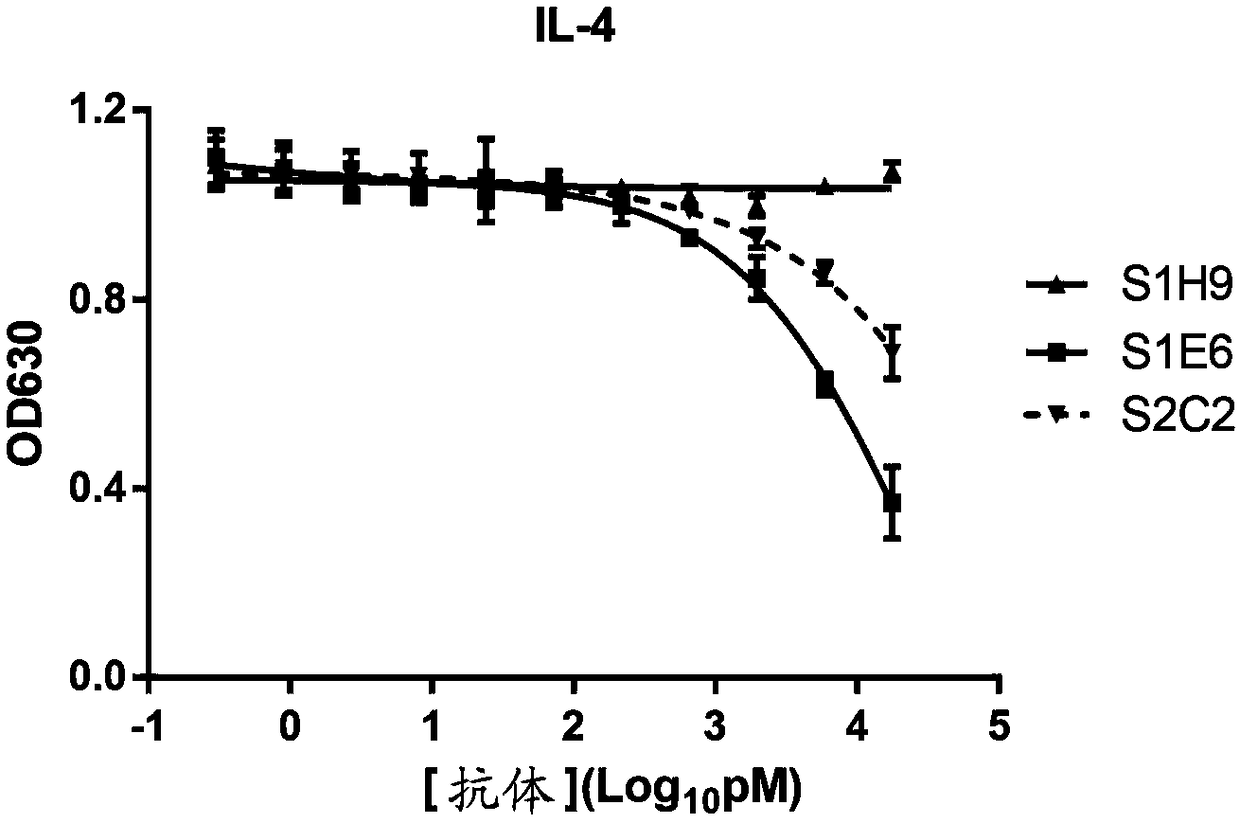
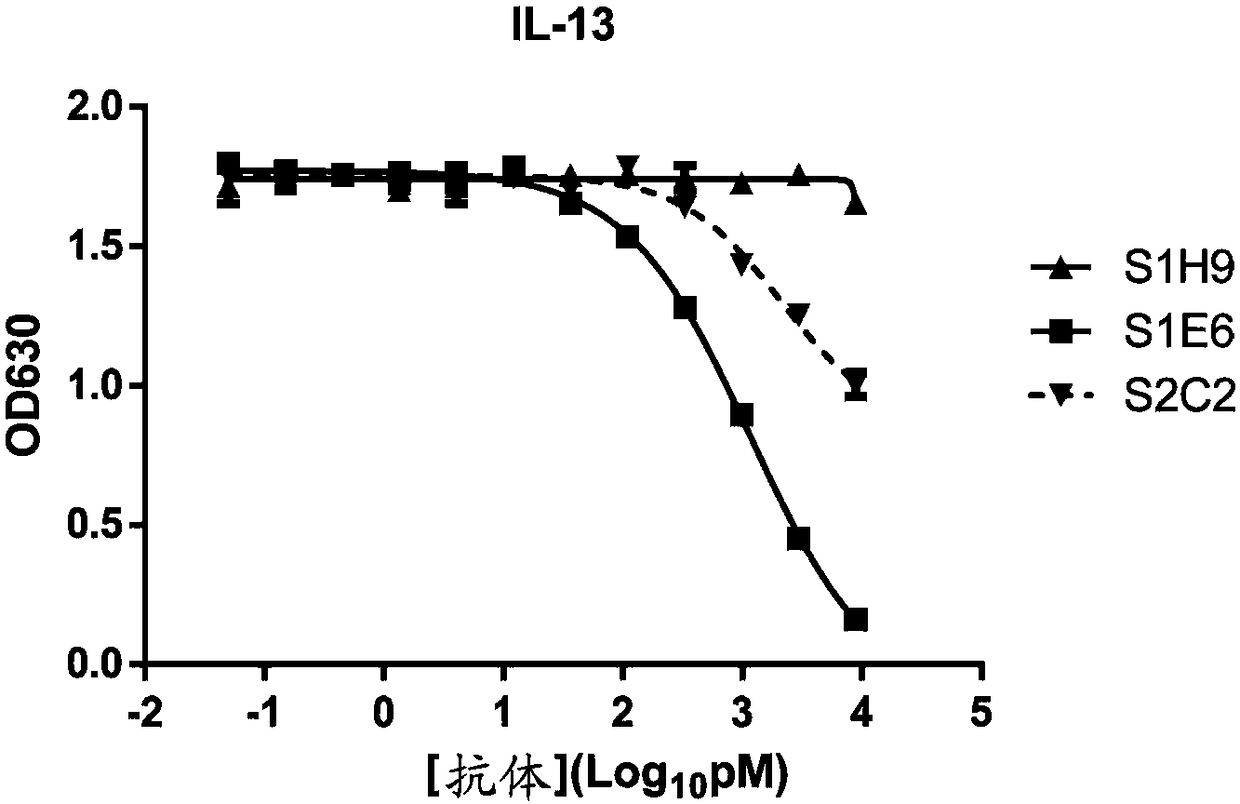
[0145] Using the recombinant hIL4R-his prepared in Example 2 as an antigen, a solid-phase screening strategy was used (for the experimental scheme, refer to Phage Display: General Experimental Guidelines / (US) Clarkson (Clackson, T.), (US) Loman (Lowman, H.B. ) edited; translated by Ma Lan et al. Chemical Industry Press, 2008.5) screened the phage library displaying the human single-chain antibody library prepared in Example 1, and obtained 3 human antibodies with different sequences but all capable of specifically binding to human IL4R, including Clone S1E6 (amino acid sequence is SEQ ID NO: 17, VH sequence is SEQ ID NO: 18, VL sequence is SEQ ID NO: 19), S1H9 (amino acid sequence is SEQ ID NO: 20, VH sequence is SEQ ID NO: 21 , VL sequence is SEQ ID NO:22), S2C2 (amino acid sequence is SEQ ID NO:23,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com