A kind of preparation method of phosphate-ligustrazine microporous osmotic pump controlled-release tablet
A technology of osmotic pump controlled release and ligustrazine, applied in the field of osmotic pump, can solve the problems of complex technology of osmotic pump preparation, difficult industrialization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of phosphoric acid-ligustrazine microporous osmotic pump controlled-release tablet, comprising the steps of:
[0030] S1. Preparation of the tablet core: the prescription composition of the tablet core is as follows:
[0031] Prescription A tablet core: Ligustrazine phosphate 100mg, sodium citrate 40mg (30mg, 40mg, 50mg, 60mg), lactose monohydrate 320mg (330mg, 320ng, 310mg, 300mg), 20% of the prescription amount of PVPK30 (15%, 18%, 20%, 22%), magnesium stearate 4.6mg;
[0032] Prescription B tablet core: Ligustrazine phosphate 100mg, sodium tartrate 60mg (40mg, 60mg, 80mg, 100mg), lactose monohydrate 300mg (320mg, 300mg, 280mg, 260mg), PVP K30 20% 20% (15% , 18%, 20%, 22%), magnesium stearate 4.6mg.
[0033] Preparation method: Mix phosphoric acid-ligustrazine, sodium citrate or sodium tartrate, and lactose monohydrate through a 60-mesh sieve, add 20% PVP K30 (95% ethanol) solution binder, make soft material, and dry at 4°C , adding whole granu...
Embodiment 2
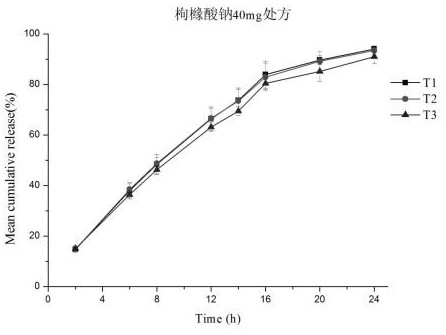
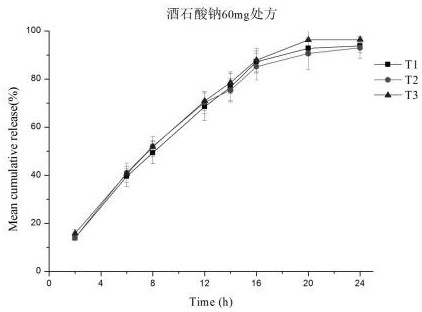
[0040] Measure the drug release capacity of the phosphoric acid-ligustrazine microporous osmotic pump controlled-release tablet prepared in Example 1 according to the routine test method in this area, and its drug release curve is as follows figure 1 with 2 shown. In addition, the fitting results of the in vitro release model are shown in Table 1 and Table 2.
[0041] Table 1. In vitro release model fitting results (formulation A)
[0042]
[0043] Table 2. In vitro release model fitting results (formulation B)
[0044]
[0045] Three batches of samples were prepared in the pilot scale-up test, and the reproducibility of the 24h release in vitro was investigated, and the release curves were fitted. Results The similarity of the release curves of two formulations and three batches of samples f 2 Between 84 and 97, the batch-to-batch reproducibility is good. The drug release model was fitted to the release curves of the same batch of preparations. The zero-order relea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com