A Calculation Method of Maximum Negative Mixing Enthalpy of Ternary Amorphous Alloy
A calculation method and technology of amorphous alloys, applied in the field of amorphous alloys, can solve the problems of complex calculation and no guiding significance of composition design, and achieve the effect of avoiding waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A calculation method for the maximum negative mixing enthalpy of a Zr-Cu-Al alloy, the specific steps are as follows:
[0030] A known component (Zr 46 ) content while the other two elements (Cu 54-x al x ) is calculated for the Zr-Cu-Al ternary alloy system with unknown content, and its molecular formula is Zr 46 Cu 54-x al x ;
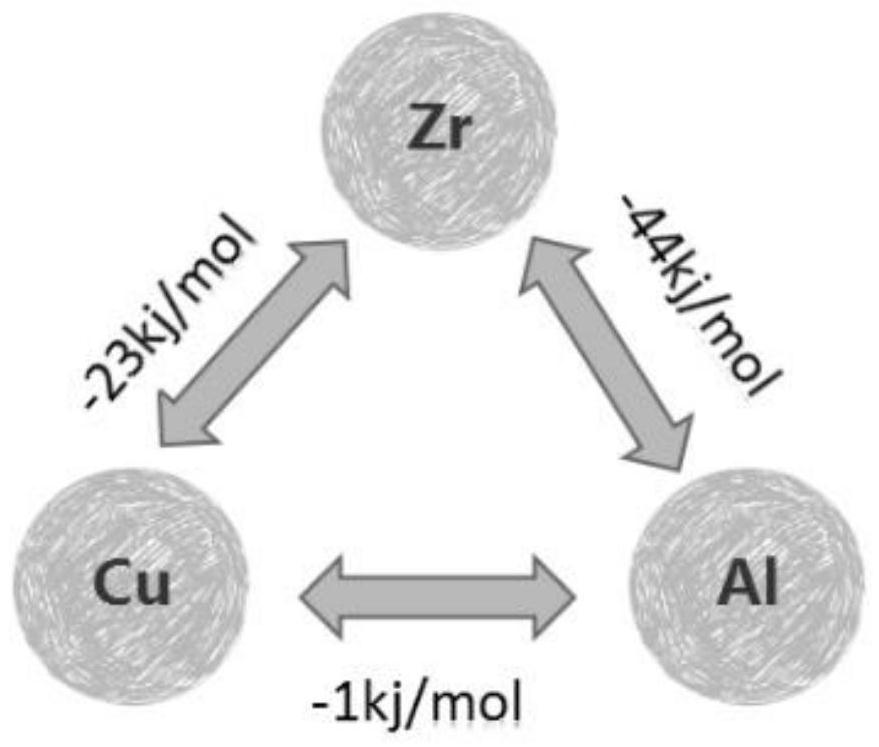
[0031] It is known that the mixing enthalpy of Cu-Zr between Zr (atomic radius 145pm)-Cu (atomic radius 117pm)-Al (atomic radius 118pm) is -23kj / mol, the mixing enthalpy of Zr-Al is -44kj / mol, and Cu The enthalpy of mixing between -Al is -1kj / mol, such as figure 1 shown.
[0032] in 1 mol of Zr 46 Cu 54-x al x Among them, the mixing enthalpy H formed when x Al atoms combine with x Zr atoms and x Cu atoms respectively 1 =x×(-44)+x×(-1)=-45xkj / mol;
[0033] There are two situations for the mixing enthalpy generated by the remaining (46-x) Zr atoms and (54-2x) Cu atoms:
[0034] (1) When (46-x)≥(54-2x), from 46-x>0, 54-2x>0, 8≤x21 =(...
Embodiment 2
[0038] A calculation method for the maximum negative mixing enthalpy of Mg-Cu-Y alloy, the specific steps are as follows:
[0039] A known component (Zr 65 ) content while the other two elements (Cu 35-x al x ) content of unknown Mg-Cu-Y ternary alloy system to calculate the mixing enthalpy, its molecular formula is Mg 65 Cu 35-x Y x ;
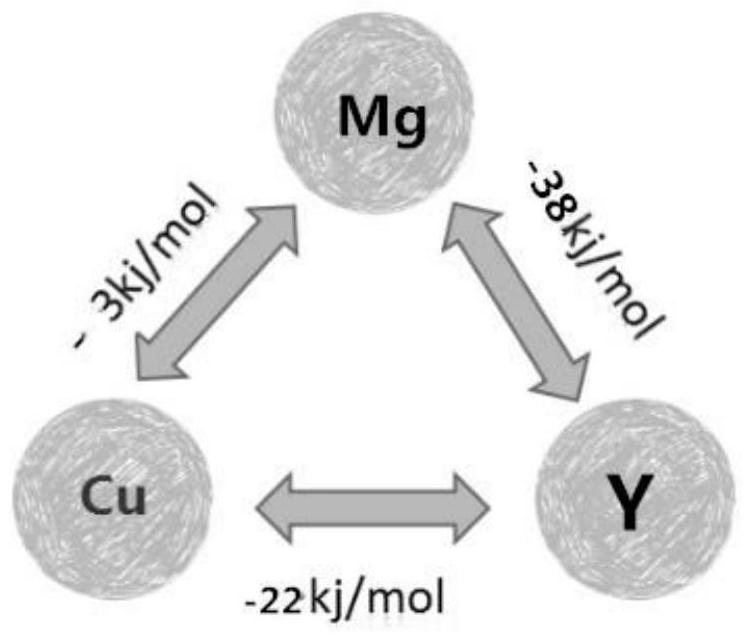
[0040] It is known that the mixing enthalpy of Cu-Mg between Mg (atomic radius 136pm)-Cu (atomic radius 117pm)-Y (atomic radius 162pm) is -3kj / mol, the mixing enthalpy of Mg-Al is -38kj / mol, and Cu The enthalpy of mixing between -Y is -22kj / mol, such as figure 2 shown.
[0041] in 1 mol of Mg 65 Cu 35-x Y x Among them, the mixing enthalpy H formed when x Y atoms combine with x Mg atoms and x Cu atoms respectively 1 =x×(-38)+x×(-22)=-60xkj / mol;
[0042] The mixing enthalpy generated by the remaining (65-x) Mg atoms and (35-2x) Cu atoms is as follows:
[0043] Because (65-x)≥(35-2x) is always established, from 65-x>0, 35-2x>0, when...
Embodiment 3
[0046] A calculation method for the maximum negative mixing enthalpy of Cu-Zr-Ti alloy, the specific steps are as follows:
[0047] A certain composition is known (Cu 60 ) content while the other two elements (Zr 40-x Ti x ) of Cu-Zr-Ti ternary alloy system with unknown content is calculated for mixing enthalpy, and its molecular formula is Cu 60 Zr 40-x Ti x ;
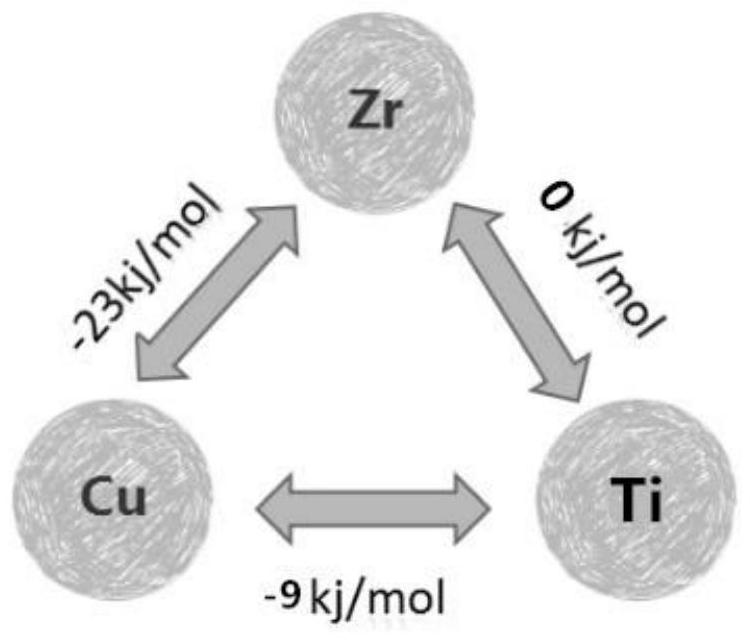
[0048] It is known that the mixing enthalpy of Cu-Zr between Cu (atomic radius 117pm)-Zr (atomic radius 145pm)-Ti (atomic radius 132pm) is -23kj / mol, the mixing enthalpy of Zr-Ti is 0kj / mol, and Cu- The enthalpy of mixing between Ti is -9kj / mol, such as image 3 shown in .
[0049] at 1mol Cu 60 Zr 40-x Ti x Among them, the mixing enthalpy H formed when x Ti atoms combine with x Zr atoms and x Cu atoms respectively 1 =x×(-9)=-9xkj / mol;
[0050] The mixing enthalpy generated by the remaining (60-x) Cu atoms and (40-2x) Zr atoms is as follows:
[0051] Because (60-x)≥(40-2x), from 60-x>0, 40-2x>0, when 02 =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com