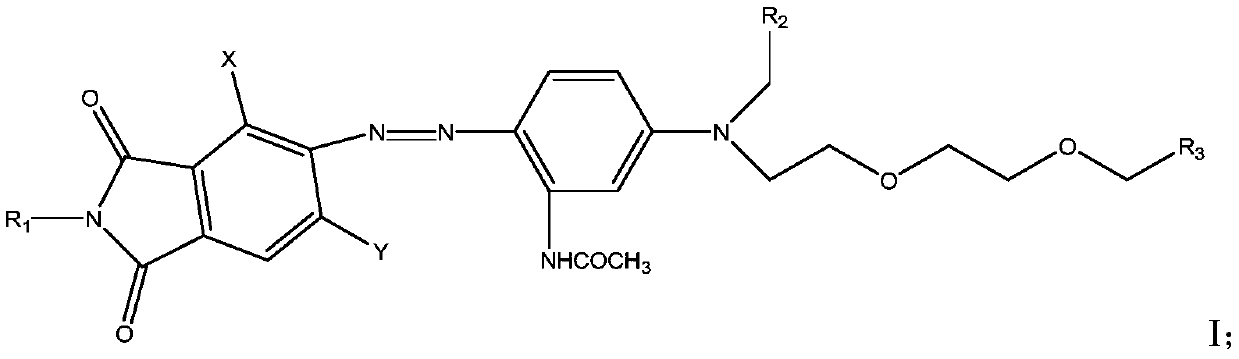
A kind of azo dye compound containing acetamide group and its preparation method and application
An azo dye and acetamide group technology is applied in the field of azo dye compounds and their preparation, which can solve the problems of low color fastness index and the like, and achieve the effects of sublimation color fastness, good comprehensive performance and bright color and light.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] step 1
[0036]
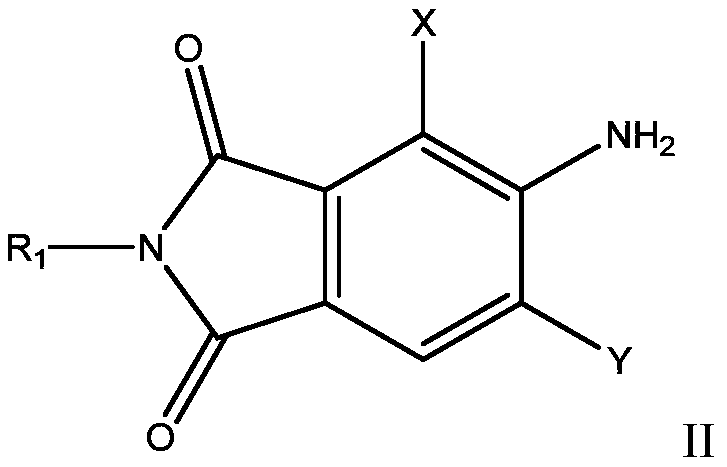
[0037] Add 112g of sulfuric acid into a 150ml three-necked flask, stir, slowly add 27g of the compound of formula II-1 below 60°C, stir until clarified, then cool down to 5-10°C and slowly add 31.5g of nitrosyl sulfuric acid (40%), add Incubate the diazo at 5-10°C for 3 hours and wait for coupling.
[0038] step 2
[0039]
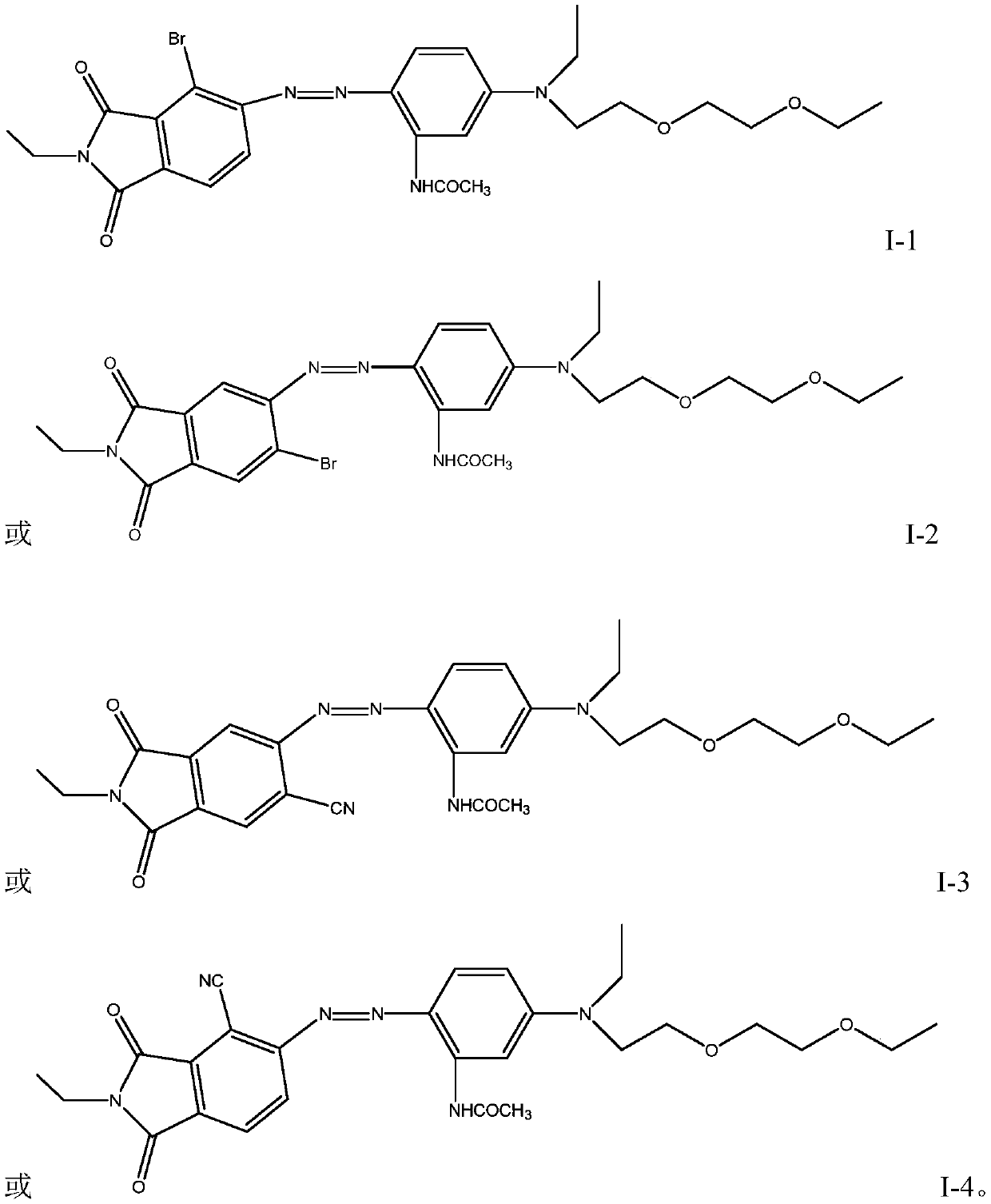
[0040] Add 500g of ice-water mixture into a 1000ml beaker, stir, add 2.5g of sulfamic acid, slowly add 29g of the compound of formula (III-1) at 5°C, and then slowly add the coupling components of the above step 1, and finish adding in 10 The temperature was kept at about ℃ for 5-6 hours, and the temperature was directly raised to 65°C, stirred, kept for 1 hour, filtered, washed with water until neutral, and separated by column chromatography to obtain 45 g of the compound of formula (I-1), with an HPLC purity of >90%. 1 H-NMR (DMSO, 300MHz): 1.15 (9H, m), 2.03 (3H, s), 2.64 (2H, t), 3.40 (2H, m), 3.46 (2H, m), ...
Embodiment 2
[0042]
[0043] According to the similar method of Example 1, the compound of formula (I-2) was prepared, and the HPLC purity was >90%. 1 H-NMR (DMSO, 300MHz): 1.15 (9H, m), 2.03 (3H, s), 2.64 (2H, t), 3.40 (2H, m), 3.46 (2H, m), 3.52 (4H, m) , 3.63(4H,m), 4.18(2H,t), 6.79(1H,d), 7.05(1H,d), 7.83(1H,d), 8.37(1H,d), 8.55(1H,d), 10.2 (1H, s).
Embodiment 3
[0045]
[0046] According to the similar method of Example 1, the compound of formula (I-3) was prepared, and the HPLC purity was >99%. 1 H-NMR (DMSO, 300MHz): 1.15 (9H, m), 2.03 (3H, s), 3.40 (2H, m), 3.46 (2H, m), 3.52 (4H, m), 3.63 (4H, m) , 4.18 (2H, t), 6.79 (1H, d), 7.05 (1H, d), 7.83 (1H, d), 8.02 (1H, d), 8.23 (1H, d), 10.2 (1H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com