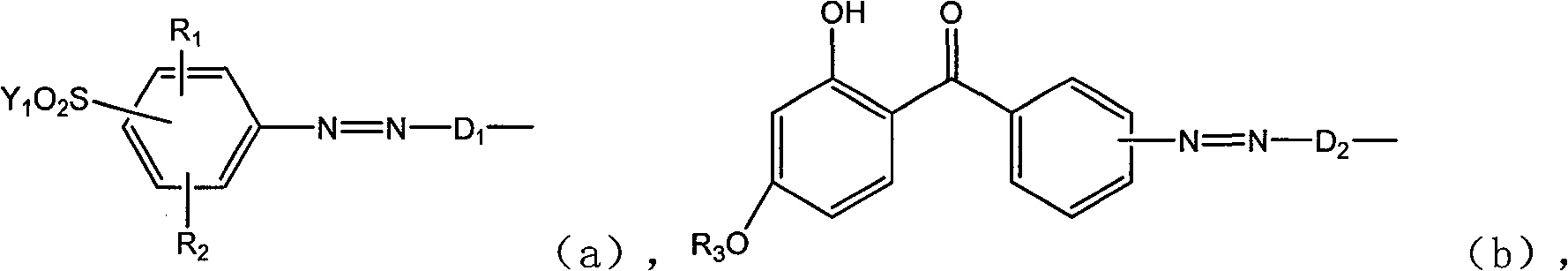
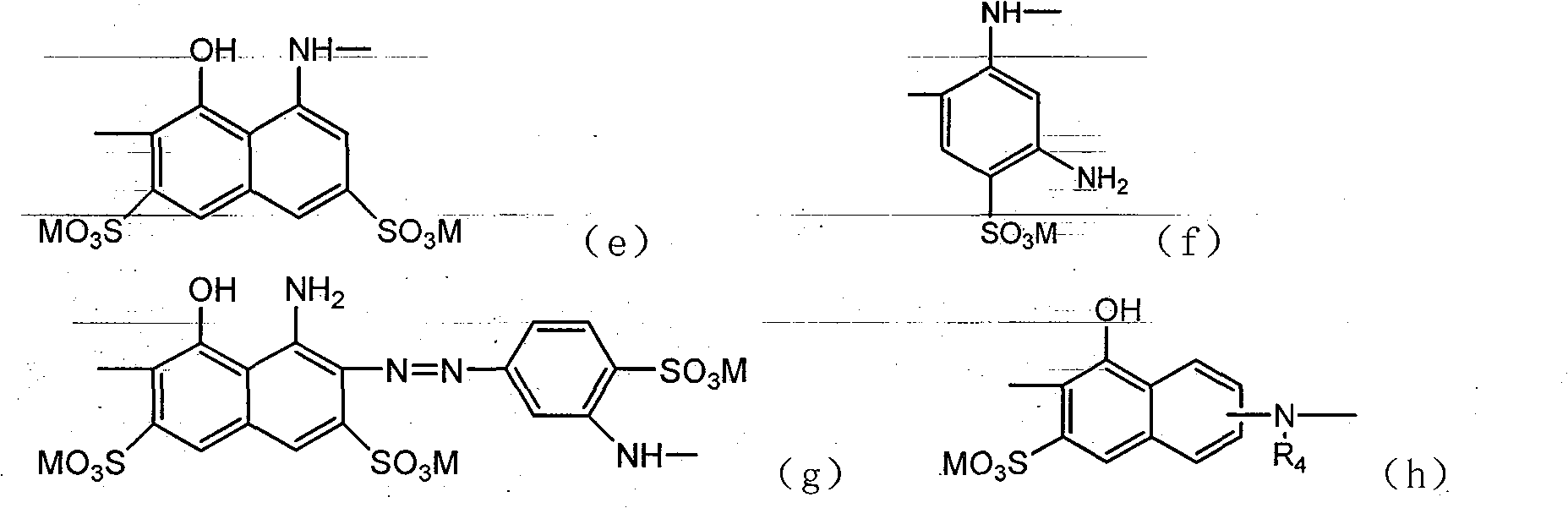
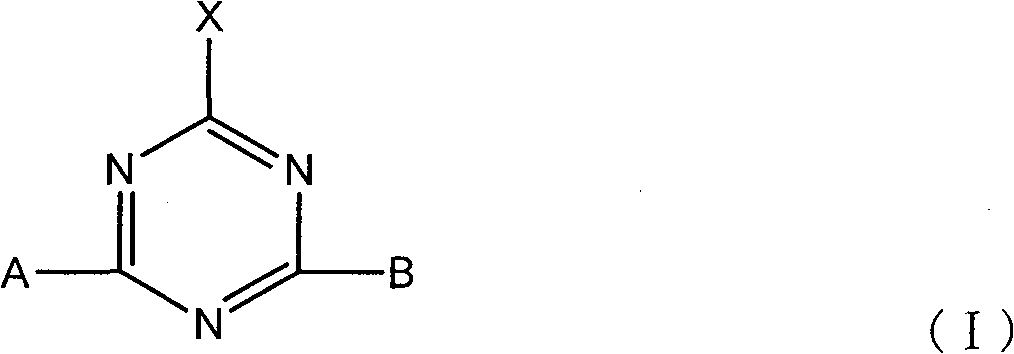Active dye containing ultraviolet absorbing groups
An external absorption, amino group-containing technology is applied in the field of reactive dyes with ultraviolet absorbing groups and their preparation, which can solve the problems of decreased use efficiency of ultraviolet absorbers, fabric discoloration, large loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Preparation of 2,4-dihydroxy-3'-aminobenzophenone:
[0124] Add 8.35g (0.05mol) of m-nitrobenzoic acid and 20ml of chlorobenzene into a 100ml three-necked flask, start stirring, dropwise add 6.248g (0.0525mol) of thionyl chloride, then add 0.5ml of DMF, heat up to 90°C for reflux reaction 2h. The resulting m-nitrobenzoyl chloride reaction solution was poured into a constant pressure dropping funnel for subsequent use.
[0125] Add 6.675g (0.05mol) of anhydrous AlCl to a 100ml three-necked flask 3 , 5.5g (0.05mol) of resorcinol, 40ml of chlorobenzene, warming up to 80°C, adding 0.5ml of pyridine, and adding the m-nitrobenzoyl chloride prepared above dropwise. After the dropwise addition, the temperature was raised to 110° C. for 5 h. After the reaction, add 30ml of water and 5ml of concentrated hydrochloric acid, stir at 100°C for 1h until there is no solid attached to the flask wall, and filter. Put the filter cake into a beaker filled with 100ml of water, add NaOH ...
Embodiment 2
[0129] Synthesis of 2-hydroxy-4-methoxy-3'-aminobenzophenone:
[0130] Add 8.35g (0.05mol) of m-nitrobenzoic acid and 20ml of 1,2-dichloroethane into a 100ml three-necked flask, start stirring, add 6.248g (0.0525mol) of thionyl chloride dropwise, and then add 0.5ml of DMF, The temperature was raised to 83.5°C and the reaction was refluxed for 2h. Pour the obtained m-nitrobenzoyl chloride reaction solution into a constant pressure dropping funnel for use.
[0131] Add 6.675g (0.05mol) anhydrous AlCl3, 7.59g (0.055mol) m-xylylene dimethyl ether, 60ml 1,2-dichloroethane into a 100ml three-necked flask, cool to 0-5°C in an ice bath, and add dropwise to prepare A good m-nitrobenzoyl chloride solution was heated to 70°C for 5 hours after the dropwise addition. After cooling, the reaction solution was slowly poured into a beaker filled with 200 g of crushed ice and 10 ml of concentrated hydrochloric acid, and stirred while pouring. Stir for 1 h until the solution is separated. Th...
Embodiment 3
[0135] Synthesis of 2-hydroxy-4-ethoxy-3'-aminobenzophenone:
[0136] In a three-necked flask, add 12.95g (0.05mol) 2,4-dihydroxy-3'-nitrobenzophenone, 60ml acetone, 5.723g (0.0525mol) CH 3 CH 2 Br, 4.74g (0.06mol)K 2 CO 3 , 0.3g KI. Reflux at 56.5°C for 48h. After filtration, the filtrate was rotary evaporated and dried to obtain 13.6 g of khaki solid 2-hydroxy-4-ethoxy-3'-nitrobenzophenone.
[0137] In the autoclave, add above-mentioned 5.75g (0.02mol) of 2-hydroxy-4-ethoxy-3'-nitrobenzophenone, 0.26g Pd / c (5%) catalyst, 100ml ethanol. Replace the air in the kettle with nitrogen for 3 times, then replace the nitrogen with hydrogen for 3 times, react at room temperature of 0.5KPa for 4h, and filter. The filtrate was rotary evaporated to obtain 5.25 g of khaki solid 2-hydroxy-4-ethoxy-3'-aminobenzophenone.
[0138]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com