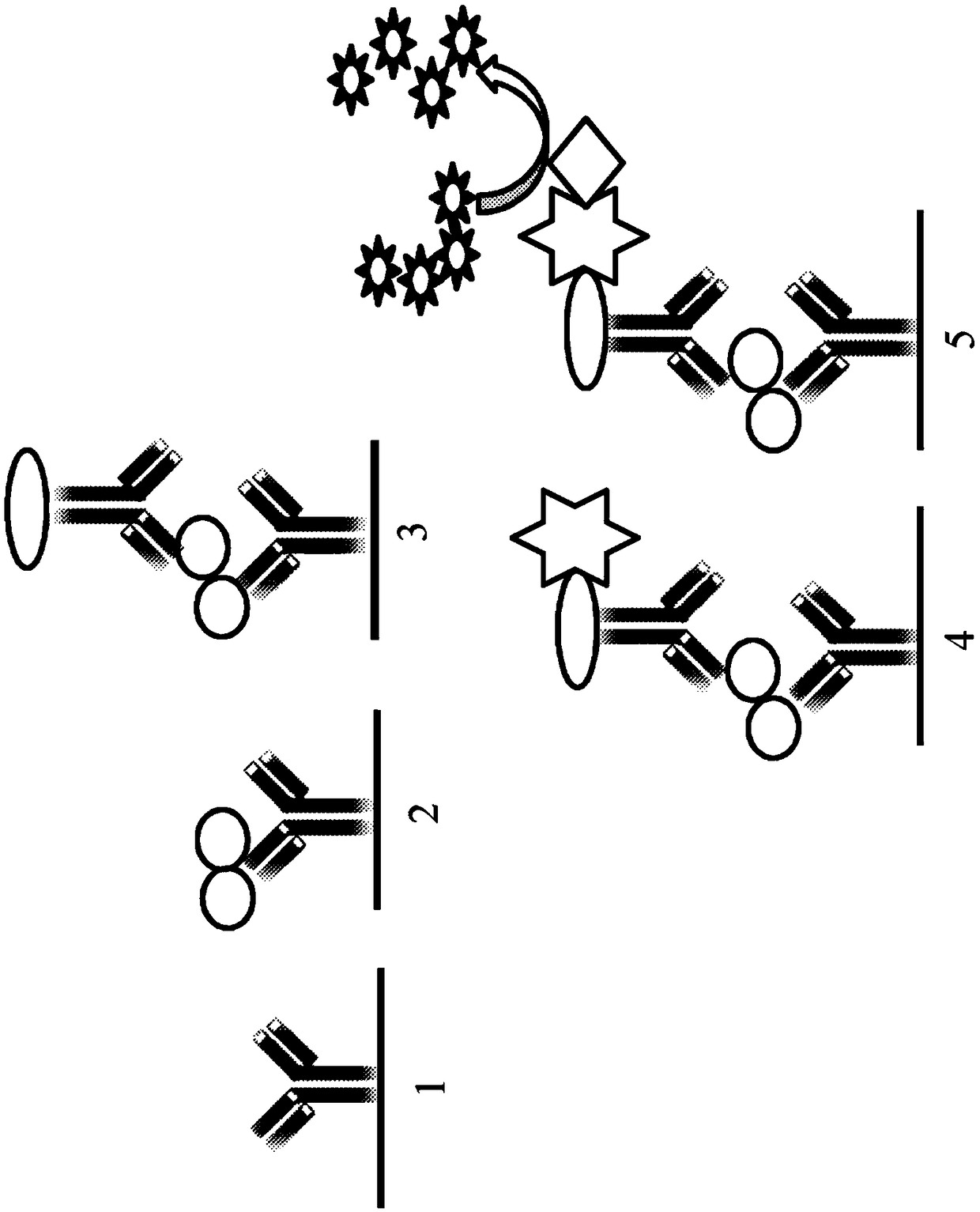
Determination of non-human mammal tk1 protein levels
A non-human mammal, TK1 technology, applied in the field of kits for determining the TK1 protein level of non-human mammals, can solve the problem of no immunochemical method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
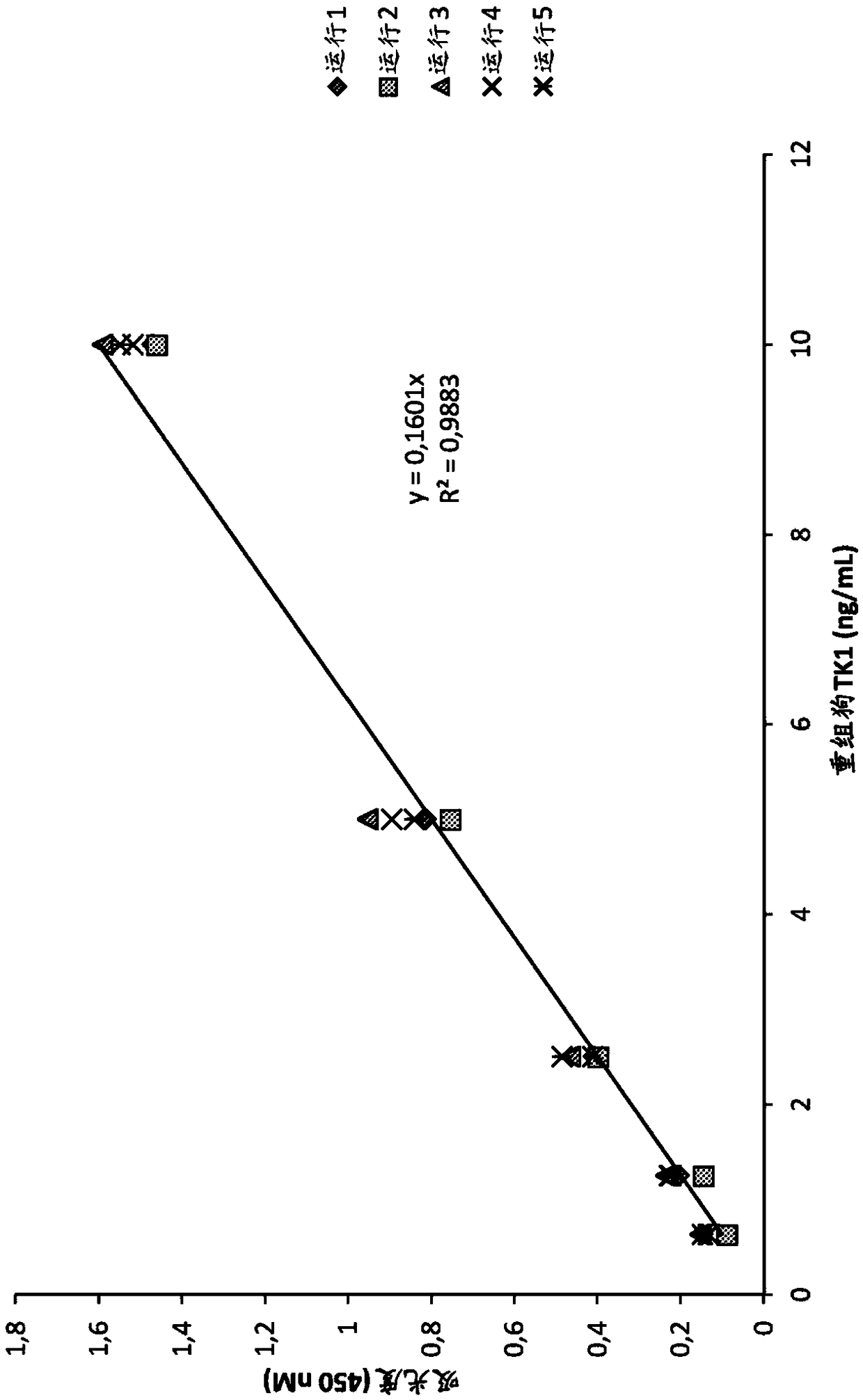
[0119] A new TK1-ELISA was developed for the determination of serum TK1 (STK1 ) protein levels in dogs with hematological and solid tumors. Both STK1 activity and STK1 protein levels were measured in sera from healthy dogs, dogs with leukemia and lymphoma, and solid tumors. In addition, serum samples from six dogs with lymphoma were followed during treatment to test the monitoring efficiency of these two TK1 assays.
[0120] Materials and methods
[0121] serum sample
[0122] Serum samples from healthy dogs, dogs with haematological and solid tumors were collected and stored at the University Animal Hospitalat the Swedish University of Agricultural Sciences, Uppsala, Sweden At -20 °C until analysis. The project was approved by the Swedish Animal Ethics Committee. The study included samples from healthy dogs (n=30), dogs with hematological tumors (n=36: lymphoma, n=31, leukemia, n=5) and dogs with solid tumors (n=40) . Mean and median ages were 6 years (range 3 to 10 yea...
Embodiment 2
[0161] The following experiments demonstrate the reactivity of dog TK1 antisera raised to peptides from different regions of the dog and feline TK1 sequence. Except for a few amino acid differences, most of the peptide sequences of TK1 are conserved among human, dog, cat and equine TK1 sequences, see figure 1 . However, there is considerable divergence between the TK1 sequences at the C-terminus of the TK1 proteins.
[0162] TK1 antiserum
[0163] Two different polyclonal rabbit antisera were raised using peptides from the C-terminal region of dog TK1 (CTK1p-211, CTK1p-215) and one antiserum was raised from the active site region of dog TK1 (CTK1p-161) . The first canine TK1 peptide sequence (CTK1p-215) antiserum was directed against a synthetic peptide of 16 amino acids (amino acids 215-230 in dog TK1; figure 1 ) to which an N-terminal cysteine (CGKPGEGKEATGVRKLF, SEQ ID NO:5) was added. The second antiserum (CTK1p-211) uses a 15 amino acid peptide (amino acids 211-225...
Embodiment 3
[0170] The procedure in Example 1 was repeated with the following differences.
[0171] Sandwich ELISA procedure for detection of TK1 in dog serum
[0172]Maxisorp, NUNC (Denmark) microtiter plates were coated with 100 μL of CTK1p-215 anti-TK1 rabbit polyclonal antibody (4 μg / mL) in carbonate buffer (pH 9.6) and incubated overnight at 4 °C . Plates were washed using a Tecan Hydro flexible microplate washer four times with wash buffer (Tris 0.1M, pH 7.4, NaCl 0.3M, Tween 0.05% and BSA 1%). Thereafter, all wells were blocked with 5% nonfat dry milk diluted in TBST for 1 hour at room temperature. Dilute 50 μL of dog serum samples in 50 μL sample dilution buffer and pre-incubate for 1 h at room temperature. Plates were washed again and pre-incubated serum samples were added. Plates were then incubated for 2 hours at room temperature on a rocking platform. Plates were washed as above and biotinylated CTKlp-161 antibody (4 μg / mL in wash buffer) was added. After incubation for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap