A target of anti-tuberculosis mycobacterium and its application
A technology of Mycobacterium tuberculosis and target, applied in the field of cell biology, can solve the problem that the relationship between Mycobacterium tuberculosis infection and lung cancer has not been reported and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 The erythroid redoxin domain of the Mycobacterium tuberculosis secretory protein PknG
[0024] Through bioinformatics analysis, it was found that Mycobacterium tuberculosis has a segment of structure domain similar to that of eukaryotic redoxin domain, which we named as rubredoxin domain. This domain exists in the amino acid position 97-139 of the effector protein PknG of Mycobacterium tuberculosis, which can interact with host ubiquitin protein, promote the proliferation and migration of tumor cells and the intracellular survival of Mycobacterium tuberculosis, and its amino acid sequence As shown in SEQ ID NO:1. The sequence of the pknG gene is shown in Gene ID: 886397.
Embodiment 2
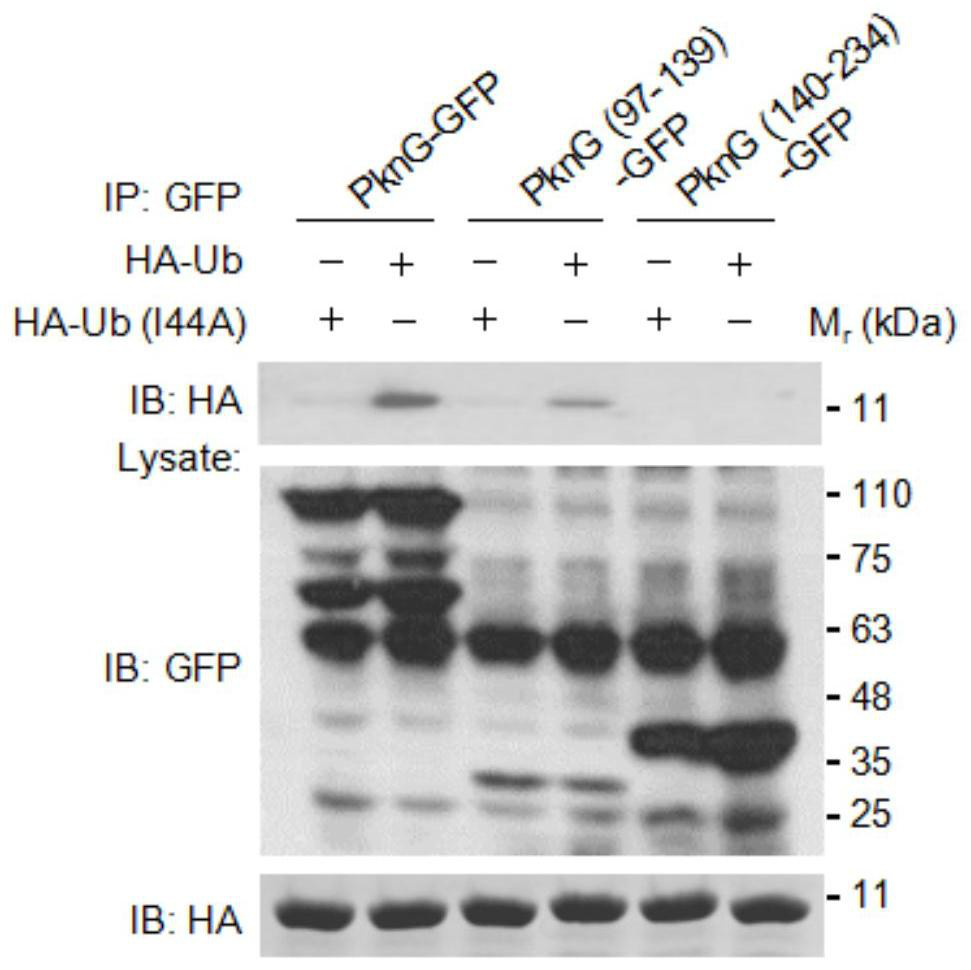
[0025] Example 2 PknG interacts with ubiquitin through the rubidoxin domain
[0026] The full-length PknG gene, the gene encoding the redoxin domain (amino acid 97-139 of PknG protein) and the gene encoding the ubiquitin-like domain (amino acid 140-234 of PknG protein) were respectively constructed into pEGFP -On the N1 plasmid; construct ubiquitin (Ub, GI number: 6647297) and its mutant Ub (I44A) (the I44 position of Ub is the key site for non-covalent hydrophobic interaction between Ub and other proteins) into PCS2 On the plasmid; use Lipofectamine 2000 (Invitrogen) to transfect the recombinant plasmid into HEK293T cells; replace the fresh medium after 6 hours of transfection; discard the medium after 24 hours of transfection, wash the cells twice with PBS, and then wash the cells with protease inhibitor The cell lysate was used to lyse the cells; the cell lysate was collected into a 1.5ml centrifuge tube and centrifuged at 13000rpm for 5 minutes; the supernatant was transfe...
Embodiment 3
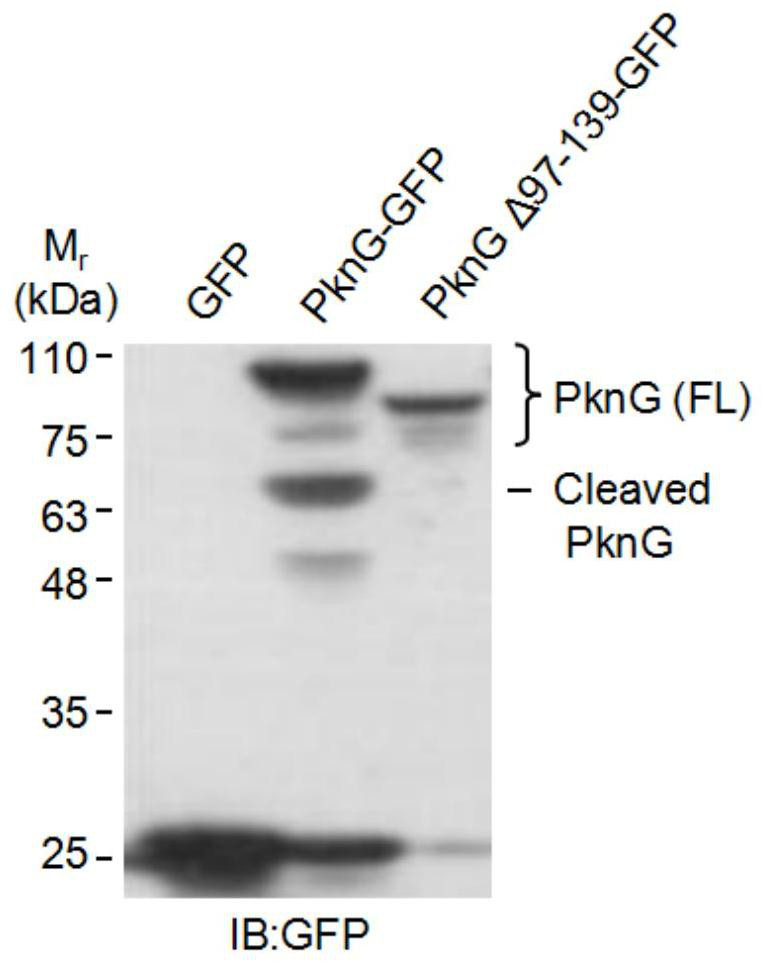
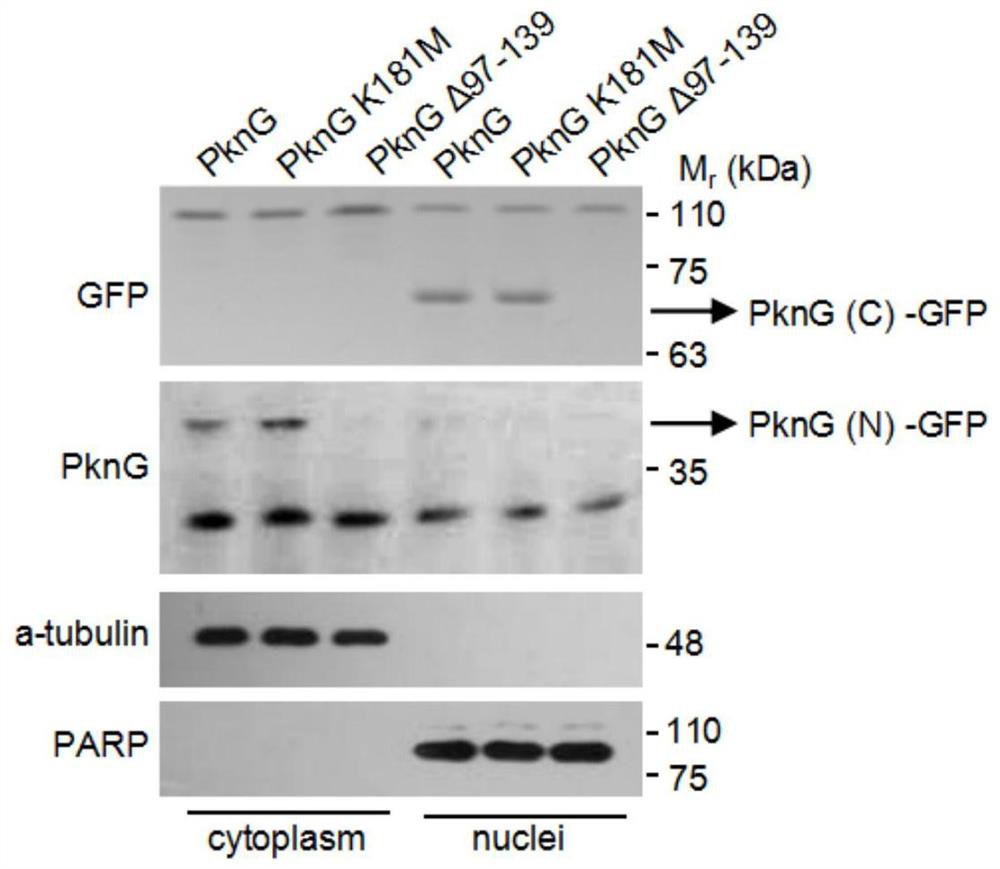
[0028] Example 3 PknG activates self-shearing function by interacting with Ub
[0029] The full-length gene encoding PknG and the deletion gene of the redoxin domain (PknG△97~139) were respectively constructed on the pEGFP-N1 plasmid; figure 2 Transfect the indicated cells into HEK293T cells; replace the fresh medium 6 hours after transfection; discard the medium 24 hours after transfection, wash the cells twice with PBS, and then lyse the cells with a cell lysate containing protease inhibitors; collect the cells Centrifuge the lysate into a 1.5ml centrifuge tube at 13000rpm for 5 minutes; transfer the supernatant to a new 1.5ml centrifuge tube, add 20 microliters of GFP-Nanoab-Agarose respectively; collect Beads by centrifugation at 4 degrees for 4 hours, The cell lysate was washed 3 times; SDS-PAGE loading buffer was added; after heating in boiling water for 10 minutes, the sample was loaded and detected by Western Blotting.
[0030] The result is as figure 2 As shown, i...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap