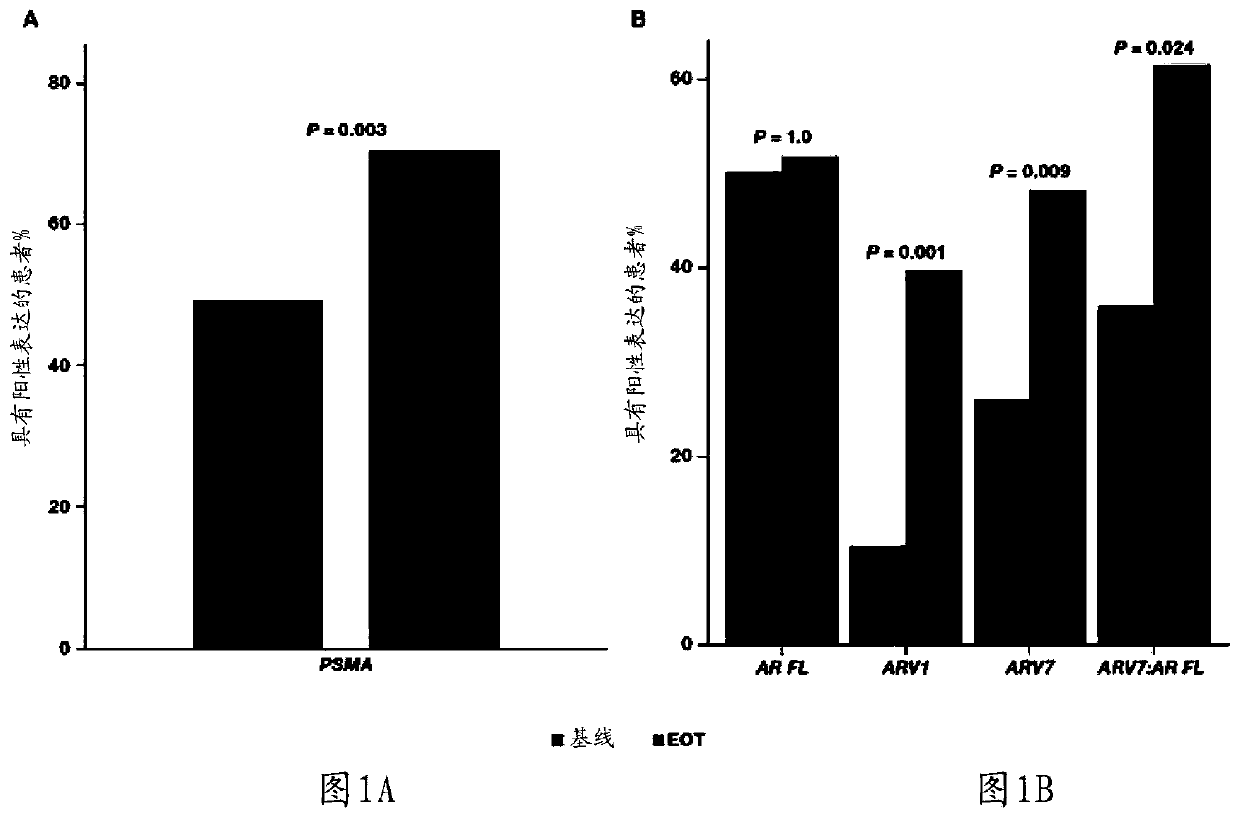
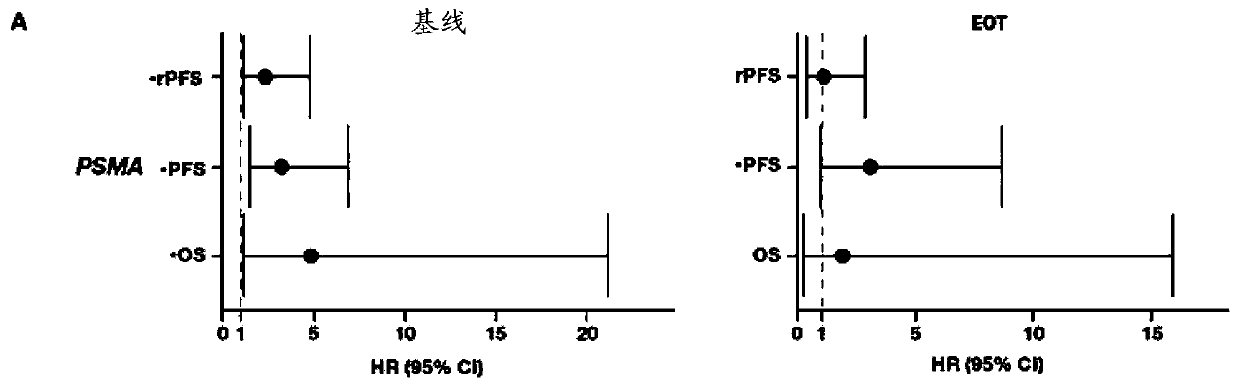
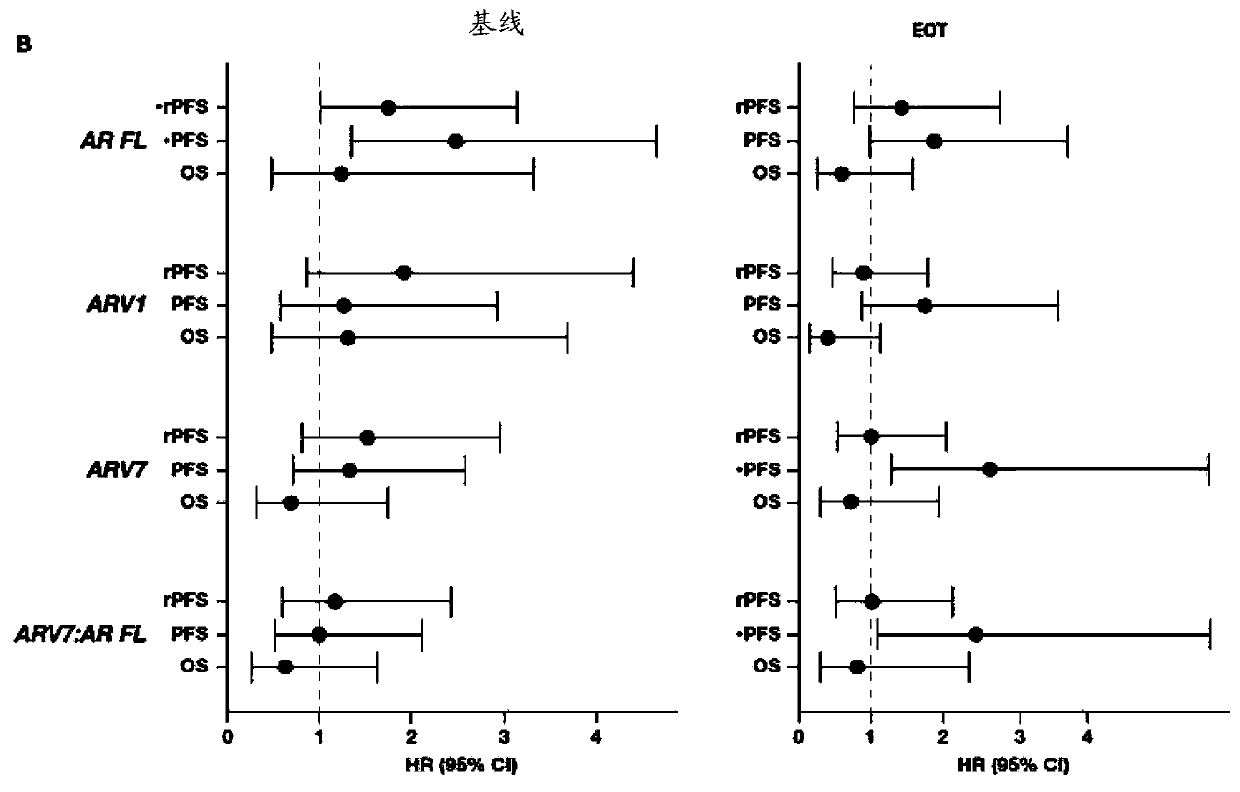
Methods of diagnosing and treating abiraterone acetate- glucocorticoid -resistant or -sensitive metastatic castration resistant prostate cancer
A technology of abiraterone acetate and glucocorticoids, applied in the field of biomarkers, can solve problems such as complex clinical decision-making
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0080] The following examples are provided to further describe some of the embodiments disclosed herein. These examples are intended to illustrate, not limit, the disclosed embodiments of the invention.
[0081] Study Design and Treatment
[0082] Two Phase 2 studies, called JPN-201 and JPN-202, were conducted in Japan. JPN-201 and JPN-202 are daily doses of 1000 mg abiraterone acetate plus 10 mg prednisone (AA) in chemotherapy-naïve (JPN-201) and chemotherapy-pretreated (JPN-202) Japanese patients with mCRPC An open-label, multicenter, single-arm Phase 2 study. Patients took orally abiraterone acetate (1000 mg, once a day) at least 1 hour before meals and 2 hours after meals at any time before 10 pm every day. In addition, prednisolone 5 mg was administered orally twice a day at the same time. The 28-day dosing cycle continued until disease progression or unacceptable toxicity was observed.
[0083] The primary endpoint was the proportion of patients achieving a PSA re...
Embodiment approach 1
[0173] Embodiment 1. A method of diagnosing and treating abiraterone acetate-glucocorticoid resistant metastatic castration-resistant prostate cancer in a patient comprising:
[0174] In a patient who has been treated with abiraterone acetate and glucocorticoids, a patient is diagnosed with abiraterone acetate when the level of PSMA, ACADL, NPY, UBE2C, or any combination thereof is elevated in said patient's biological sample Abiraterone-glucocorticoid-resistant metastatic castration-resistant prostate cancer; and
[0175] The diagnosed patient is treated with a therapeutically effective amount of a therapeutic agent other than abiraterone acetate and a glucocorticoid or with abiraterone acetate and a glucocorticoid plus a therapeutically effective amount of an additional therapeutic agent.
Embodiment approach 2
[0176]Embodiment 2. A method of diagnosing and treating abiraterone acetate-glucocorticoid sensitive metastatic castration-resistant prostate cancer in a patient comprising:
[0177] In a patient who has been treated with abiraterone acetate and glucocorticoids, the patient is diagnosed as having Abiraterone acetate-glucocorticoid-sensitive metastatic castration-resistant prostate cancer; and
[0178] The confirmed patient is treated with a therapeutically effective amount of abiraterone acetate and a glucocorticoid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com