Application of cilomilast in preparation of drug for treating symptoms associated with acute kidney injury
A technology for acute kidney injury and related diseases, applied in the field of cilomilast, can solve problems such as AKI that has not yet been
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
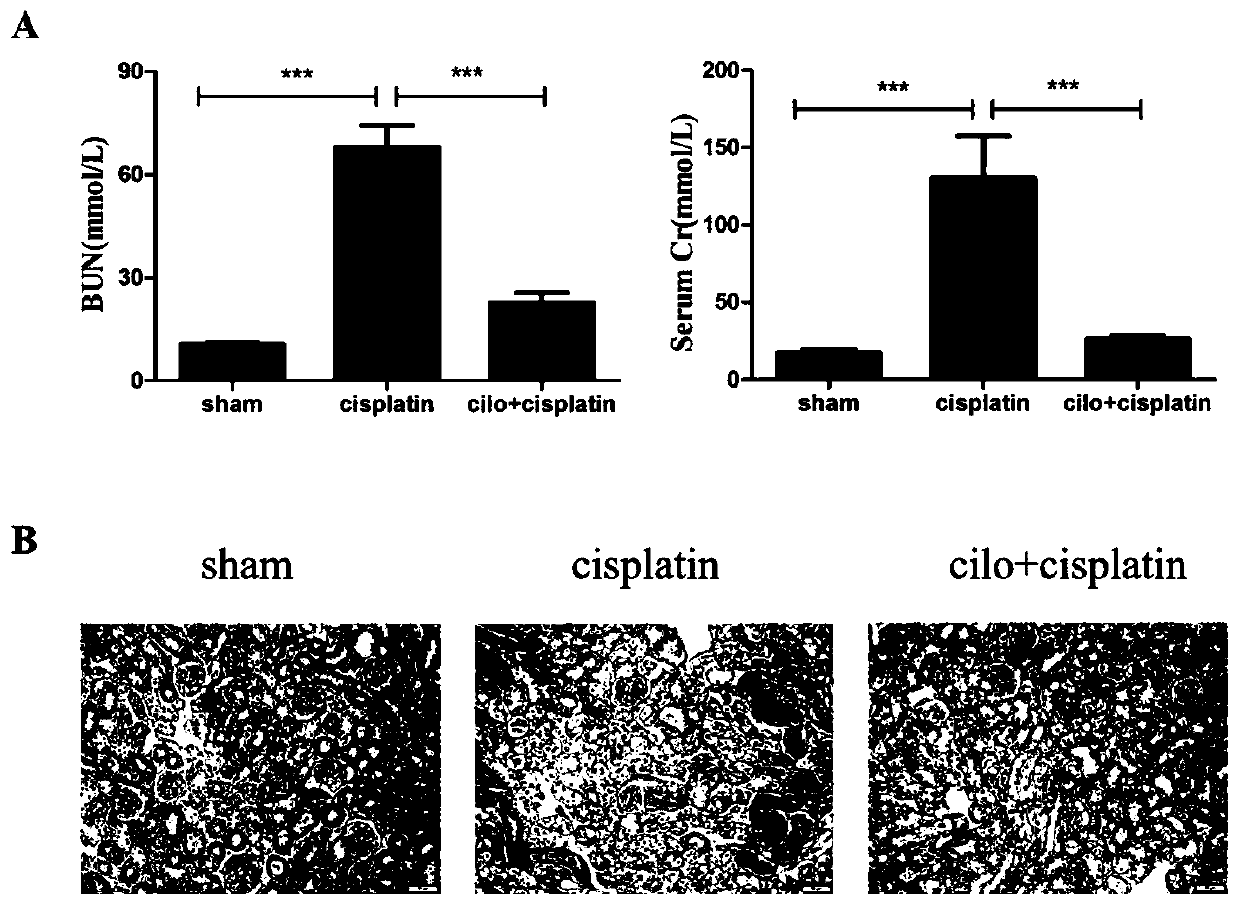
[0035] Example 1 Effect of Cilomilast on renal function in cisplatin-induced acute kidney injury.
[0036] Male C57BL / 6 mice weighing 18-22 g were taken and divided into 3 groups, namely the control group, the cisplatin model group and the Cilomilast treatment group (n=8).
[0037] Control group: intraperitoneal injection of equal volume medium once a day for 4 days in total;
[0038] Cisplatin model group: intraperitoneal injection, 20mg / kg, single administration;
[0039]Cilomilast treatment group: Cilomilast was administered for 1 day in advance (intraperitoneal injection, 30 mg / kg, Cilomilast, 200 μL / time), followed by a single administration of cisplatin (intraperitoneal injection, 20 mg / kg), Cilomilast was treated for another 3 days, and cisplatin was injected Blood was collected after 72 hours, and kidney tissue was retained.
[0040] The blood sample was centrifuged (20min, 3000r / min), and the creatinine assay kit (Creatinine Assay Kit (cat: K625-100, biomars)), urea...
Embodiment 2
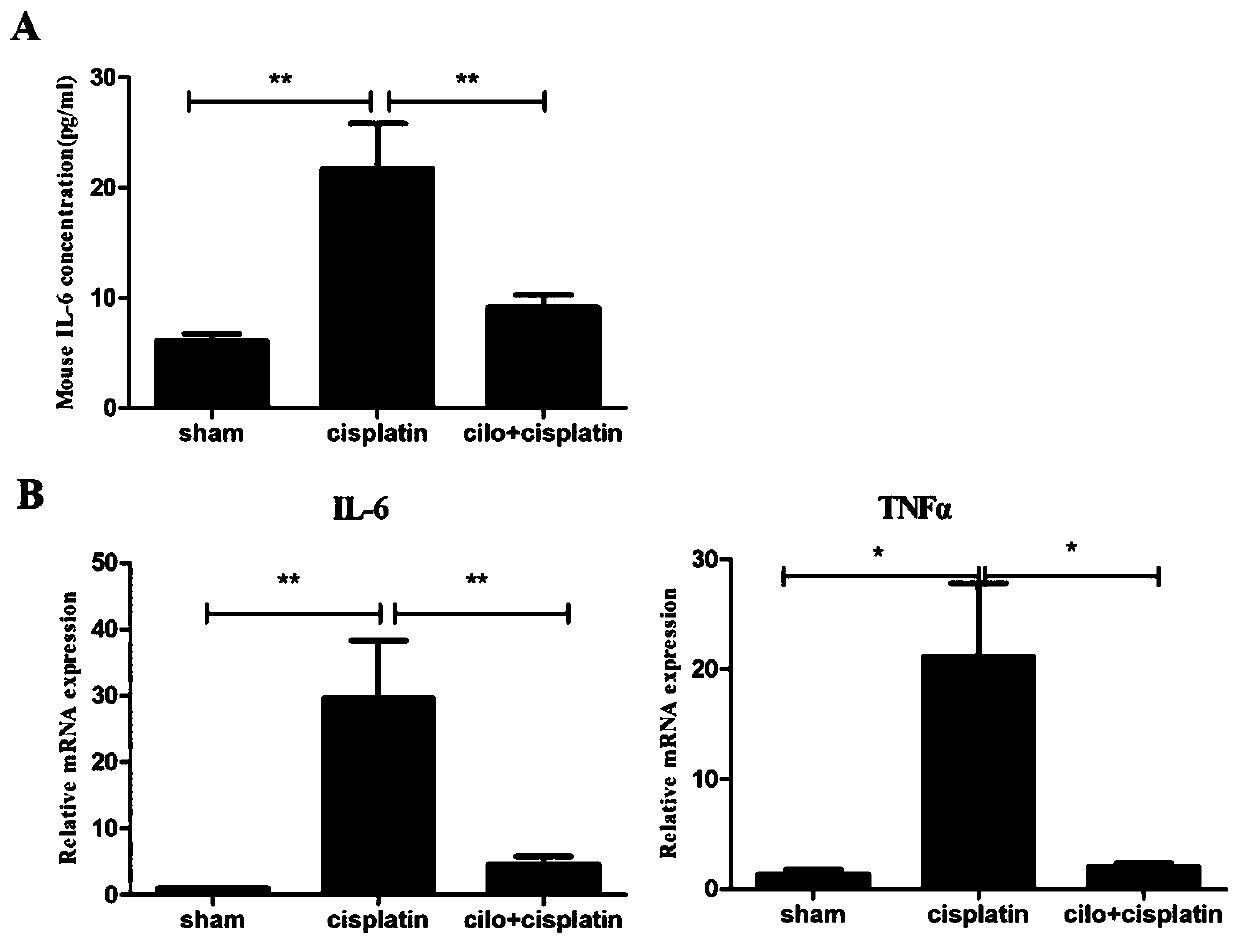
[0042] Example 2 Effect of Cilomilast on the expression and secretion of cisplatin-induced renal inflammatory molecules.
[0043] The effects of Cilomilast on the expression and secretion of cisplatin-induced renal inflammatory molecules were studied by QPCR and ELISA.
[0044] Such as figure 2 As shown in A, the effect of Cilomilast on the expression and secretion of cisplatin-induced renal inflammatory molecules was studied by ELISA method. In the cisplatin-induced AKI model, the expression level of the inflammatory factor IL-6 in the cisplatin model group was significantly higher than that in the control group, p<0.01. The Cilomilast treatment group can significantly reduce the expression level of IL-6, p<0.01.
[0045] Such as figure 2 As shown in B, the QPCR method was used to study the effect of Cilomilast on the expression and secretion of cisplatin-induced renal inflammatory molecules. In the cisplatin-induced AKI model, the expression levels of inflammatory fact...
Embodiment 3
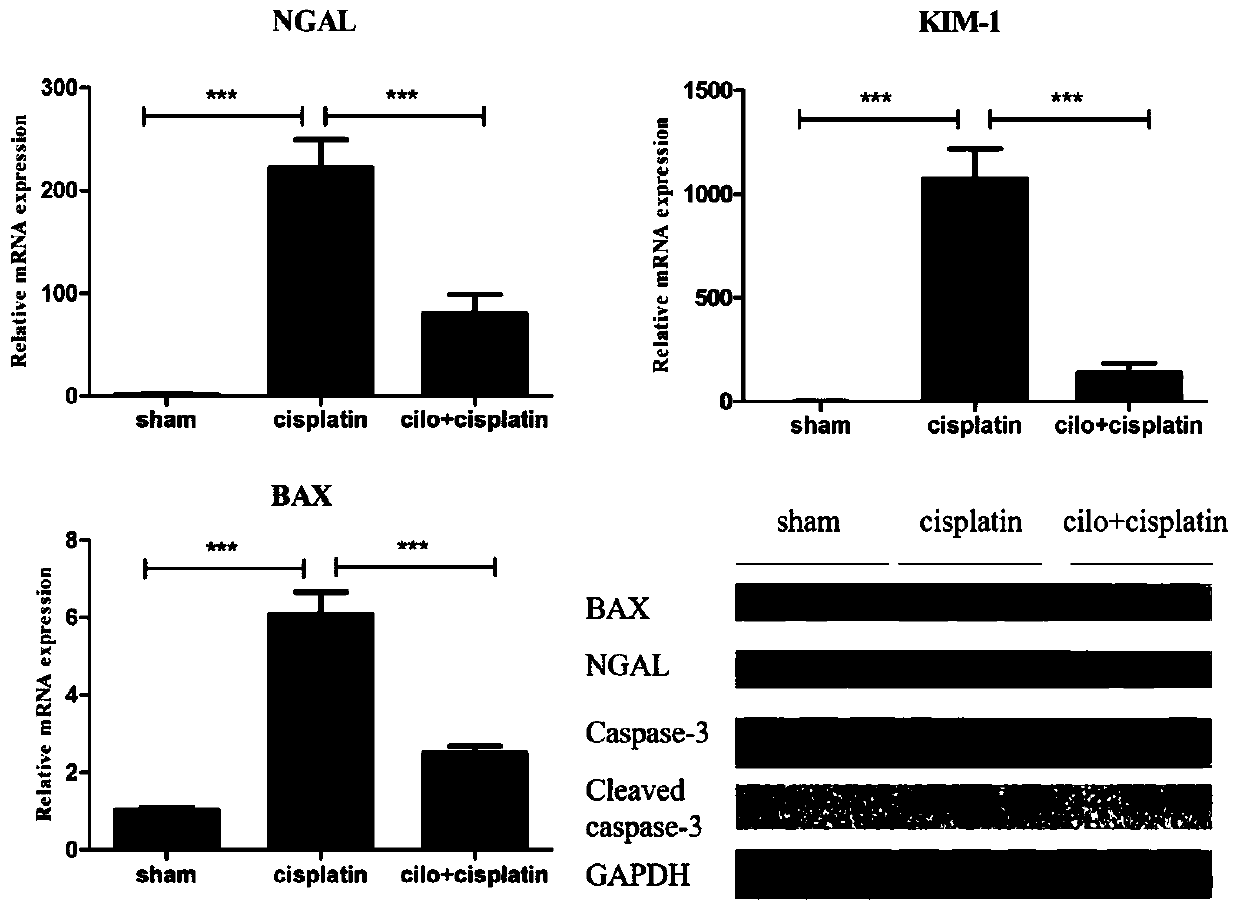
[0047] Example 3 Effect of Cilomilast on cisplatin-induced tubular cell apoptosis and kidney injury-related molecules.
[0048] QPCR and Western blot were used to detect the expression levels of kidney apoptosis and loss-related molecules to determine the improvement of Cilomilast on cisplatin-induced AKI. The results are as follows image 3 As shown, the expressions of apoptosis-related molecules Bax and Cleaved caspase 3 in the cisplatin model group were significantly increased; the expressions of damage-related molecules KIM-1 and NGAL were significantly increased. The Cilomilast treatment group can significantly reduce the expression levels of Bax, KIM-1, NGAL, and Cleaved caspase 3.
[0049] The results showed that Cilomilast could significantly reduce the expression levels of cisplatin-induced renal apoptosis and loss-related molecules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com