Compositions and methods for protein expression and delivery
A ferritin and assembly technology, applied in the field of compositions and methods for protein expression and delivery, can solve the problems of harmful proteins, harsh conditions, and difficulty in recovering proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1: Materials and methods
[0103] Plasmids and Competent Cells
[0104] Cloning was performed using pRSF1b expression vector (Merck) and pBAD / HisB (Life Technologies). Transformations were performed using chemically competent XL1Blue E. coli cells (Simply Science) and BL21(DE3) E. coli cells (Simply Science).
[0105] Reagents and Antibodies
[0106] The following reagents were used: restriction enzymes NcoI, EagI and SpeI (New England BioLabs), ExpresslinkT4 DNA ligase (Life Technologies), Luria-Bertani (LB) agar (Axil Scientific), kanamycin (ThermoFisher), ampicillin ( Axil Scientific), Omega bio-tek Plasmid Mini Kit and Gel Extraction Kit (Simply Science), Site-directed mutagenesis kit (New England BioLabs), isopropyl β-D-1-thiogalactopyranoside, IPTG (Axil Scientific), L-arabinose (Sigma), Tris-HCl pH8.0 (Sigma ), sodium chloride NaCl (Sigma), Triton-X 100 (Sigma), calcium chloride CaCl 2 (Sigma), hemin (Sigma), β-mercaptoethanol (Sigma), imidazole (...
Embodiment 2
[0156] Example 2: Engineering of a Thermally Stable Enclosure (tES)
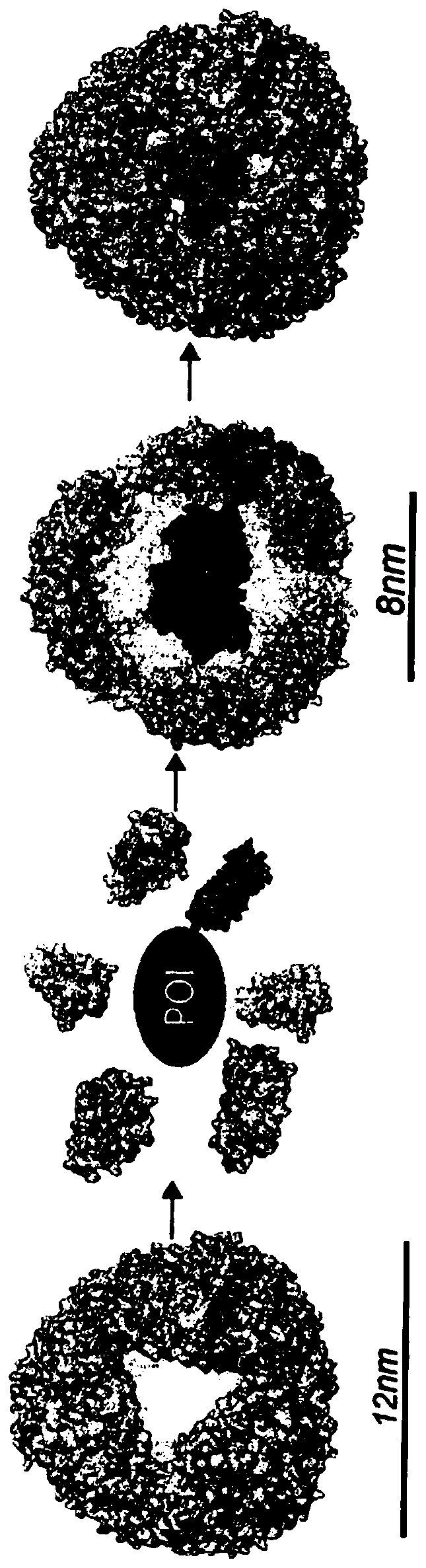
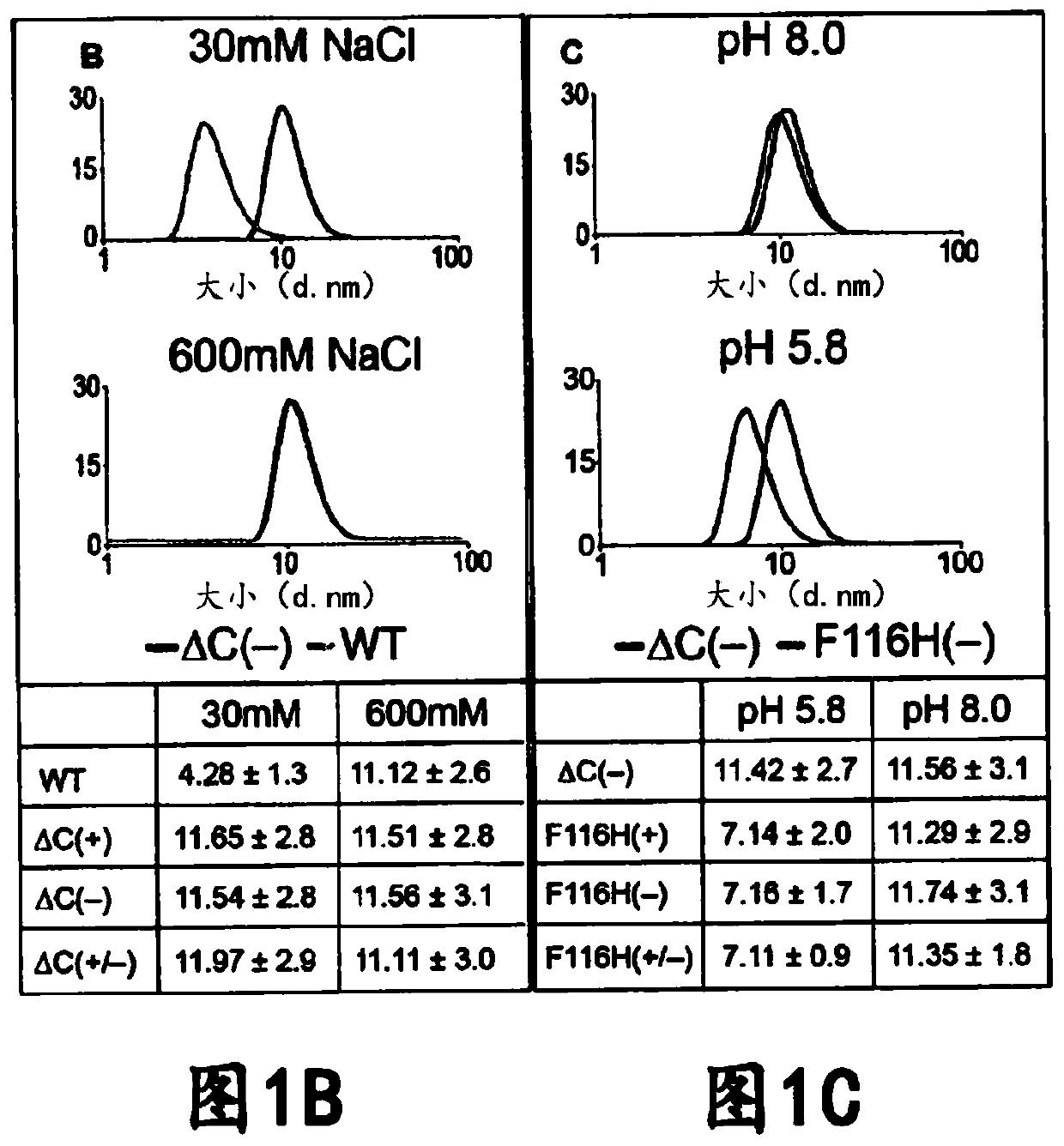
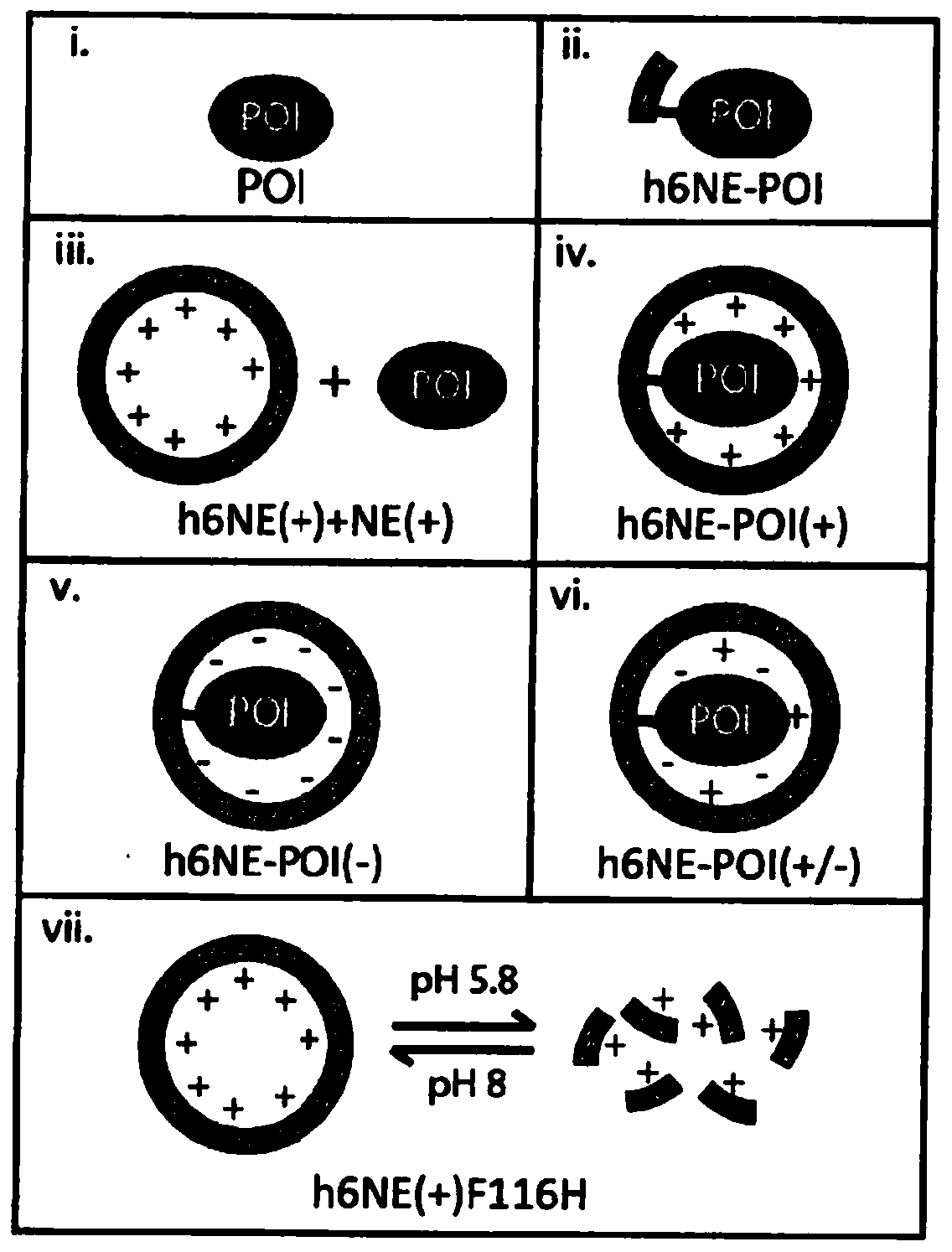
[0157] The interior of the AfFtn assembly was engineered to maximize cage volume and accommodate the folding requirements of the internalized protein. Examination of the primary sequence and crystal structure of wild-type (WT) AfFtn revealed that subunit interface residues were predominantly hydrophobic and residues in the interior were negatively charged (Johnson, E. et al., Structure. 13, 637- 648 (2005)). Altogether, approximately 25 kDa and 72 negative charges are contributed from the unstructured C-terminus of the WT subunit to the internal volume of the AfFtn assembly. The engineered NE described herein is a C-terminal truncation mutant at residue 164 ( Figures 7A-7F ). The C-terminal truncation did not affect nanocage assembly as confirmed by comparison between the truncation and the size exclusion curves of WT AfFtn. Surprisingly, analytical light scattering studies revealed that removal of the ...
Embodiment 3
[0159] Example 3: Iron uptake properties of tES
[0160] The native AfFtn coat is a physiological iron storage protein; therefore it was investigated whether tES variants also retain iron in our purified preparations or when exposed to in vitro iron loading protocols (Macara IG, et al., The Biochemical journal 126, 151-162 (1972 )). Equimolar amounts (1 μM) of tES(+), tES(-), tES(+ / -) and commercial horse spleen ferritin were compared and no detectable iron core was observed in the purified tES variants ( Figure 10A ) (Johnson, E. et al., Structure. 13, 637-648 (2005)). Then wild-type AfFtn, tES(+), tES(-), tES(+ / -), tES(+)F116H, tES(+)F116H / tES-GFPuv, tES(+) were used in equimolar amounts (1 μM) F116H / tES-HRPC and tES(+)F116H / tES-rLuc tested iron uptake. Wild-type AfFtn showed the highest in vitro iron uptake, whereas the empty tES shell had significant but lower iron accumulation, possibly due to amino acid substitutions near (E128 and E131) ferrous oxidase (Klenk HP et ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com