Fused 1,4-oxazepines as bet protein degraders
A compound and heteroaryl technology, which can be used in drug combinations, antiviral agents, medical preparations containing active ingredients, etc., can solve the problems of RNA polymerase phosphorylation and transcription output increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
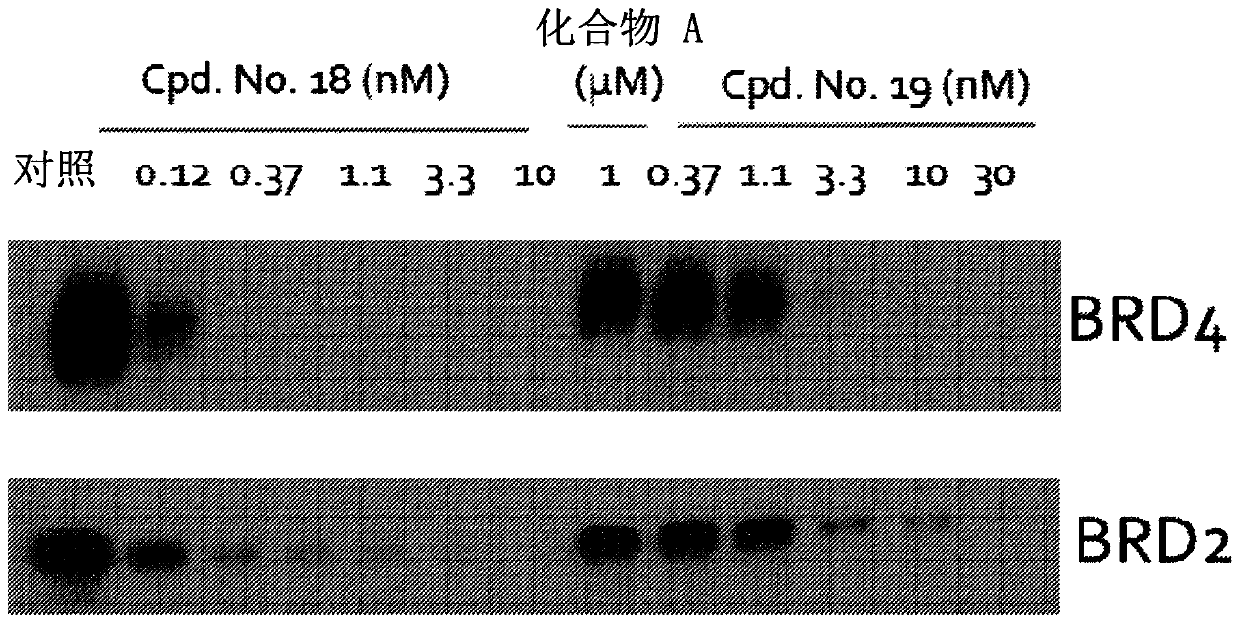
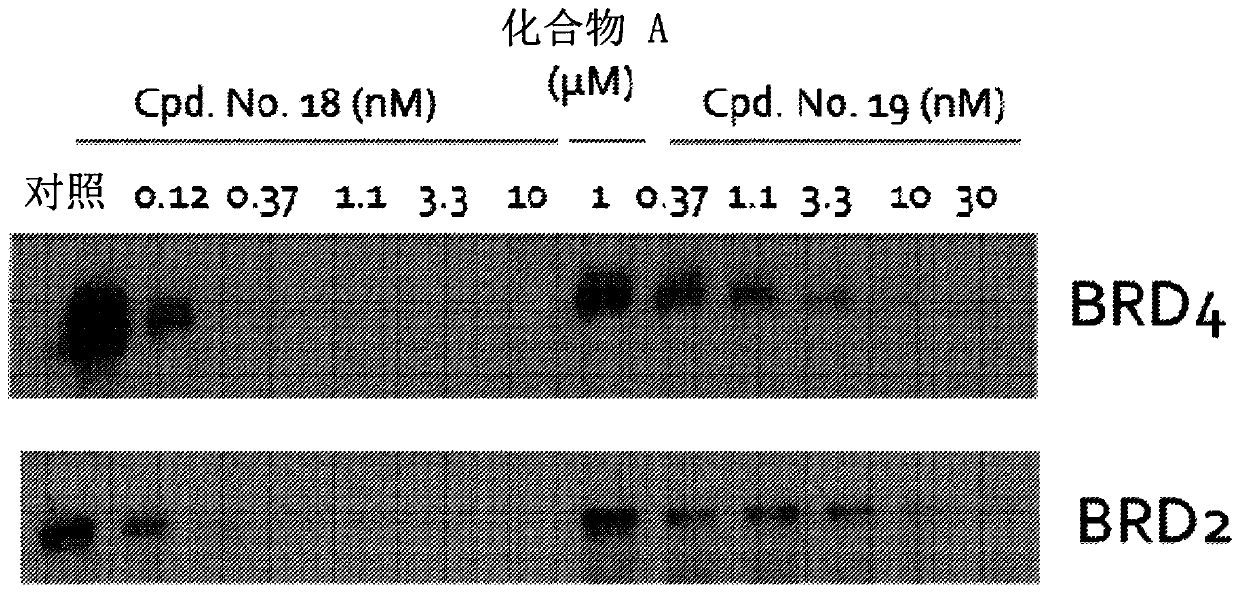
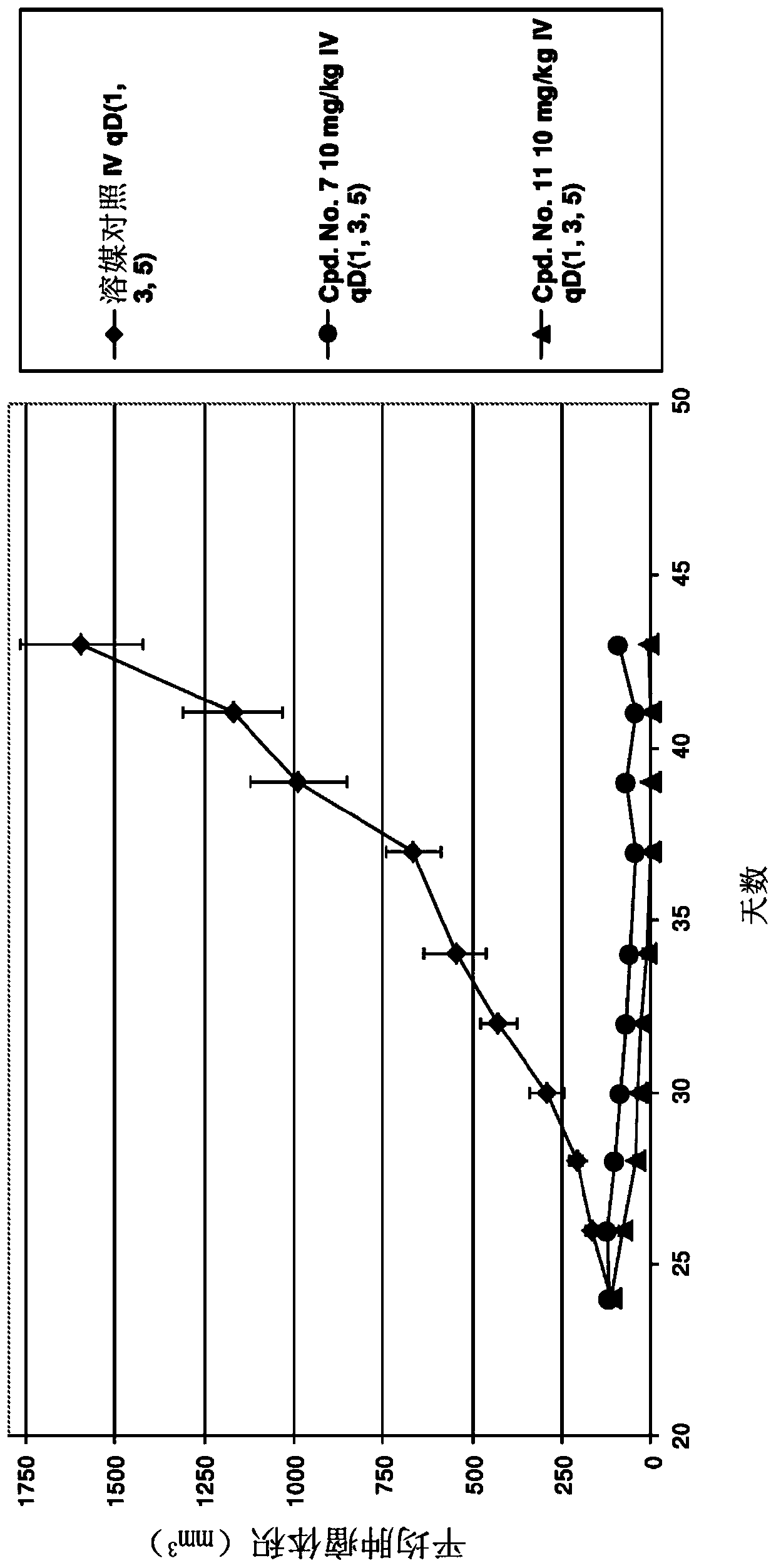
Image
Examples
preparation example Construction
[0495] This disclosure encompasses the preparation and use of salts of the disclosed compounds. As used herein, a "pharmaceutically acceptable salt" of a drug refers to a salt or zwitterionic form of a compound of the present disclosure. Salts of compounds of the present disclosure can be prepared during the final isolation and purification of the compounds or separately by reacting the compounds with an acid having a suitable cation. Pharmaceutically acceptable salts of the disclosed compounds may be acid addition salts formed with pharmaceutically acceptable acids. Examples of acids which can be employed to form pharmaceutically acceptable salts include inorganic acids such as nitric, boric, hydrochloric, hydrobromic, sulfuric and phosphoric, and organic acids such as oxalic, maleic, succinic and citric. Non-limiting examples of salts of compounds of the present disclosure include, but are not limited to, hydrochloride, hydrobromide, hydroiodide, sulfate, bisulfate, 2-hydro...
Embodiment approach I
[0541] Embodiment I: A method of treating a patient with cancer, the method comprising administering to the patient a therapeutically effective amount of a compound of the disclosure, wherein the patient's cells contain a biomarker, and the biomarker is MCL-1 Overexpression, BCL-X L Overexpression or MCL-1 and BCL-X L common overexpression.
Embodiment approach II
[0542] Embodiment II: A method of treating a patient with cancer, the method comprising:
[0543] (a) Determination of MCL-1, BCL-X in biological samples from patients L or MCL-1 and BCL-X L and when it is determined that the expression level is higher than that of a control sample (for example, a sample from a normal non-diseased patient or a sample from a patient with cancer but not expressing MCL-1, BCL-X L or MCL-1 and BCL-X L When the expression level of the patient's sample),
[0544] (b) administering to a patient a therapeutically effective amount of a compound of the disclosure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com