Novel pegylated liposomal formulations of apelin for treatment of cardiovascular-related diseases
A technology of liposomes and therapeutic agents, applied in the field of treatment of cardiovascular-related diseases and diseases, can solve problems such as short plasma half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
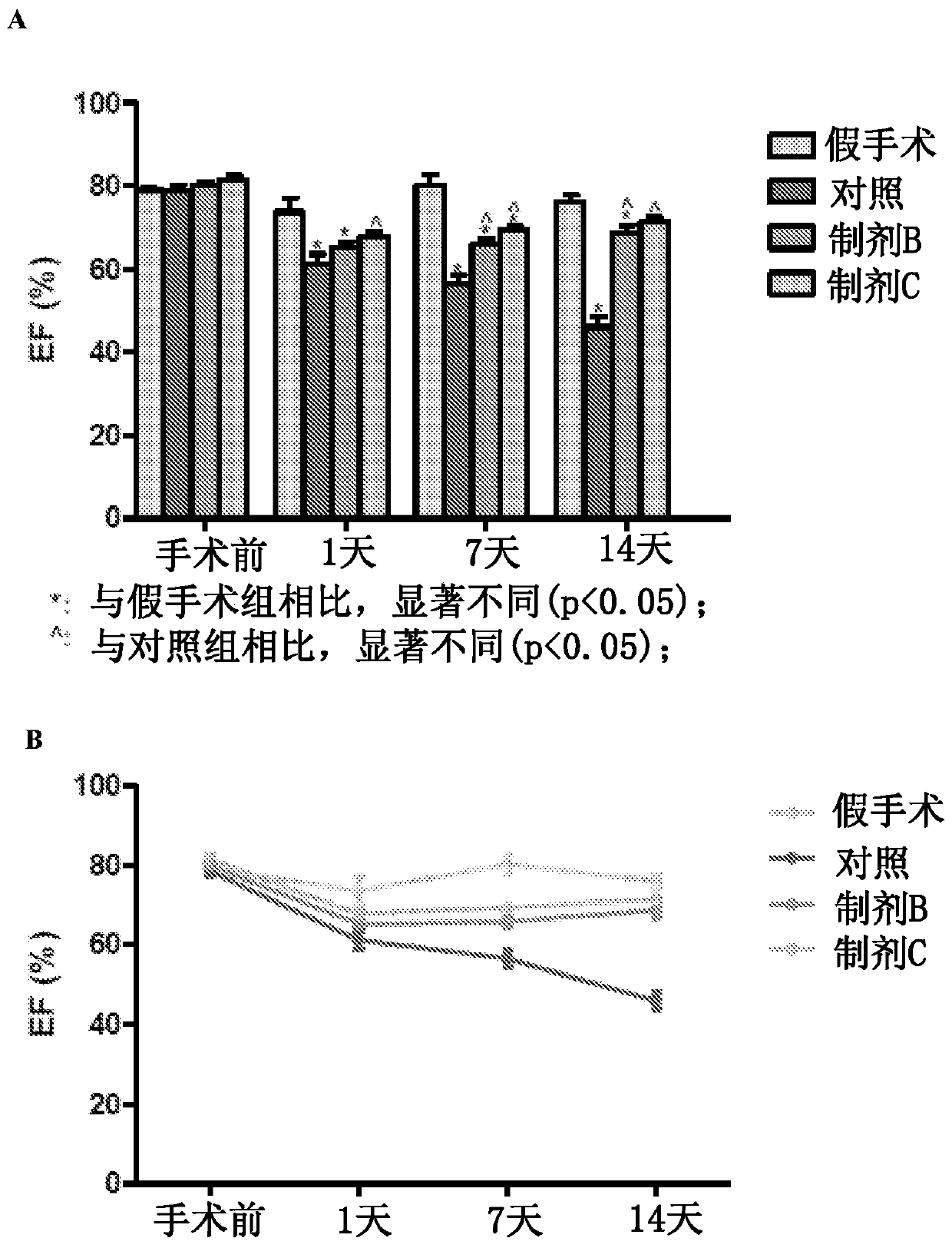
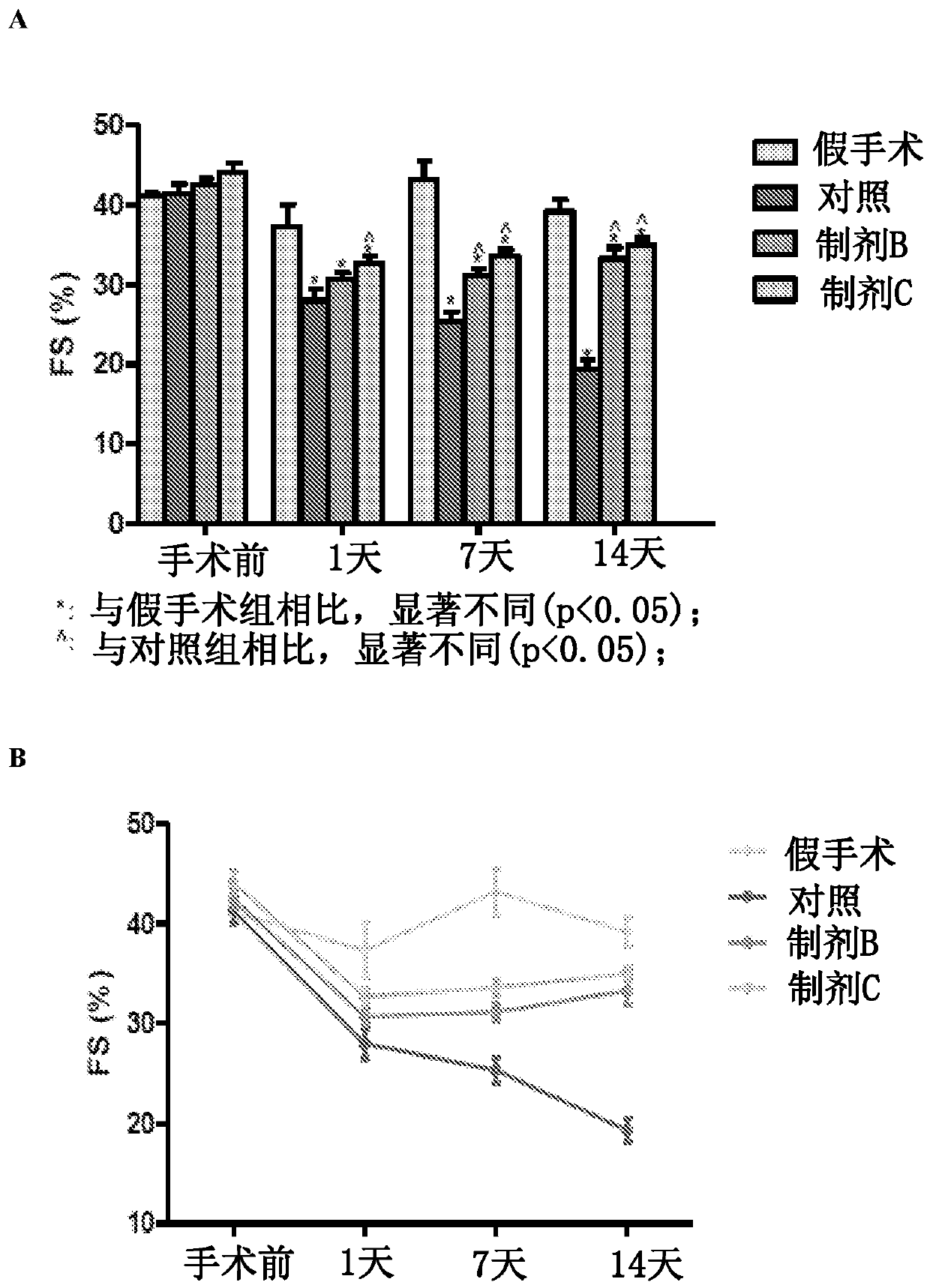
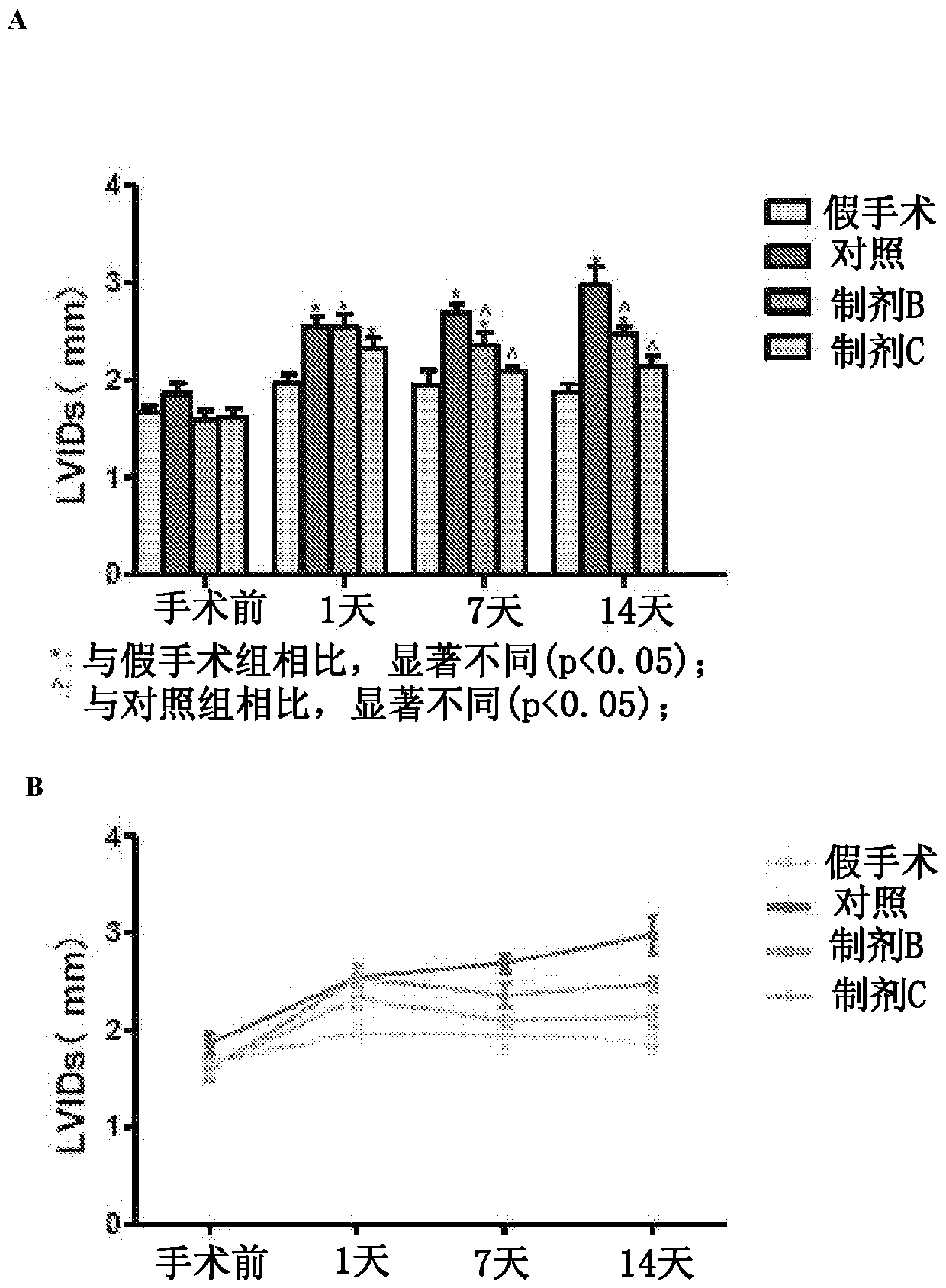
[0095] Example 1: Pharmacodynamics of Apelin Preparations in a Mouse Model of Myocardial Hypertrophy and Heart Failure
[0096] Animals. C57BL / 6J mice aged 6 to 8 weeks and 8 to 10 weeks were used as pharmacodynamic animal models of transverse aortic constriction (TAC)-induced cardiac hypertrophy and heart failure. Mice were grouped accordingly into treatment groups as shown in Table 3 below. Mice received intraperitoneal injections of 100 μL of PBS (sham group), vehicle-only (liposome) control group, apaline only (formulation B group), or apaline-liposome (formulation C group) .
[0097] Table 3: Mouse Treatment Groups
[0098] therapy group number of animals mock surgical group 6 Control group – liposomes only 6 Formulation Group B – Apelene only 7 Formulation C only – Apelin Liposomes 7
[0099] TAC procedure. Sterilize the surgical field for binocular stereoscopic manipulation. Prepare the incision to reveal the heart and left...
Embodiment 2
[0113] Embodiment 2: Apelin preparation for the treatment of myocardial infarction
[0114] Protocol for animal experiments. Male Sprague-Dawley rats (age, 8 years) weighing 180-220 g were obtained from standard sources. Rats were maintained in a temperature-controlled (20-22° C.) environment on a 12-hour light-dark cycle. Rats were divided into three groups (n=6 / group) as follows: sham operation; left anterior descending artery (LAD) ligation group; and LAD+apalene group. The Apelene group received Apelene liposomes according to the formulation illustrated in Table 4 below.
[0115] Table 4: Therapeutic formulations
[0116]
[0117] Rats in the LAD group underwent LAD ligation (Patten 1998; Ahn 2004). Briefly, rats were anesthetized with 10% chloral hydrate (3.5 ml / kg) via intraperitoneal injection. In the supine position, endotracheal intubation was performed and rats were ventilated using a rodent ventilator (rate, 80 breaths / min; tidal volume, 6-8 ml / kg). The c...
Embodiment 3
[0119] Embodiment 3: Apelene preparation is for the treatment of pulmonary arterial hypertension (PAH)
[0120] Twenty patients with PAH will participate in a randomized, double-blind, placebo-controlled study of Apelin liposomes and matching saline placebo injections during right heart catheterization (Vestbo 2013; Vestbo 2015). The Apelene group received Apelene liposomes according to the same formulation as illustrated in Table 4 above.
[0121] Measure mean pulmonary artery pressure, pulmonary artery wedge pressure, and cardiac output. Treatment with liposomal apelene is expected to result in an increase in cardiac output.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap