Bifunctional small molecule compound capable of activating enzyme catalytic reaction, and applications thereof
A small molecule compound and catalytic reaction technology, applied in organic chemistry, fermentation, etc., can solve problems such as unsatisfactory catalytic effect, achieve the effect of improving catalytic activity and stereoselectivity, and enhancing activation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] Preparation Examples of Small Molecular Compounds
[0066] 1) Synthesis of imidazolyl carboxylic acids with different chain lengths
[0067] Taking the preparation of ImC6 as an example,
[0068]
[0069] Tomko R.; Overberger, C.G.J. Polym. Sci. Pol. Chem., 1985, 23, 265–277.
[0070] Hack, S.; B.; G.; Pabel, J.; Wanner, K.T. Eur. J. Med. Chem. 2011, 46, 1483–4984.
[0071] Groves, J.T.; Neumann, R.J. Am. Chem. Soc. 1989, 111, 2900-2909.
[0072] General method 1 (taking 6-imidazolyl caproic acid as an example):
[0073] Add imidazole (1.4g, 20.0mmol) into a flask containing 15mL of toluene, slowly add sodium hydride (0.5g, 20.0mmol) while stirring, reflux at 120°C for 3h, cool to room temperature, slowly add ethyl bromohexanoate ( 2.3g, 10.0mmol) of toluene solution, reflux overnight, TLC detection of reaction progress.
[0074] After the reaction, cool to room temperature, concentrate toluene with a rotary evaporator and spin dry, add an appropriate amount...
Embodiment 1
[0131] BMP utilization (Im-C6-Phe) catalysis epoxidation reaction
[0132] The reaction system is as follows: 4mM (10% MeOH solubilization), 0.5μM enzyme BMP (PatrickC.Cirino, Frances H.Arnold.Advanced Synthesis & Catalysis, 2010,344(9):932-937.), 0.5mM Small molecules, 20mMH 2 o 2 Add to PBS (100mM) with pH 8.0 in a final volume of 1mL, react in a water bath at 25°C for 30min, add 125μL of 4-(4-nitrobenzyl)pyridine (0.4g) in absolute ethanol (10mL), 80 ℃ water bath for 30 min, then ice bath for 10 min, add 1 mL of acetone / triethylamine mixed solution (1:1). The 570nm absorption was measured with a UV spectrophotometer (Shimadzu Spectrophotometer UV-1800), and the catalytic reaction without adding small molecule compounds was used as a control (Table 1). The result of the reaction is as follows:
[0133] Table 1
[0134]
[0135]
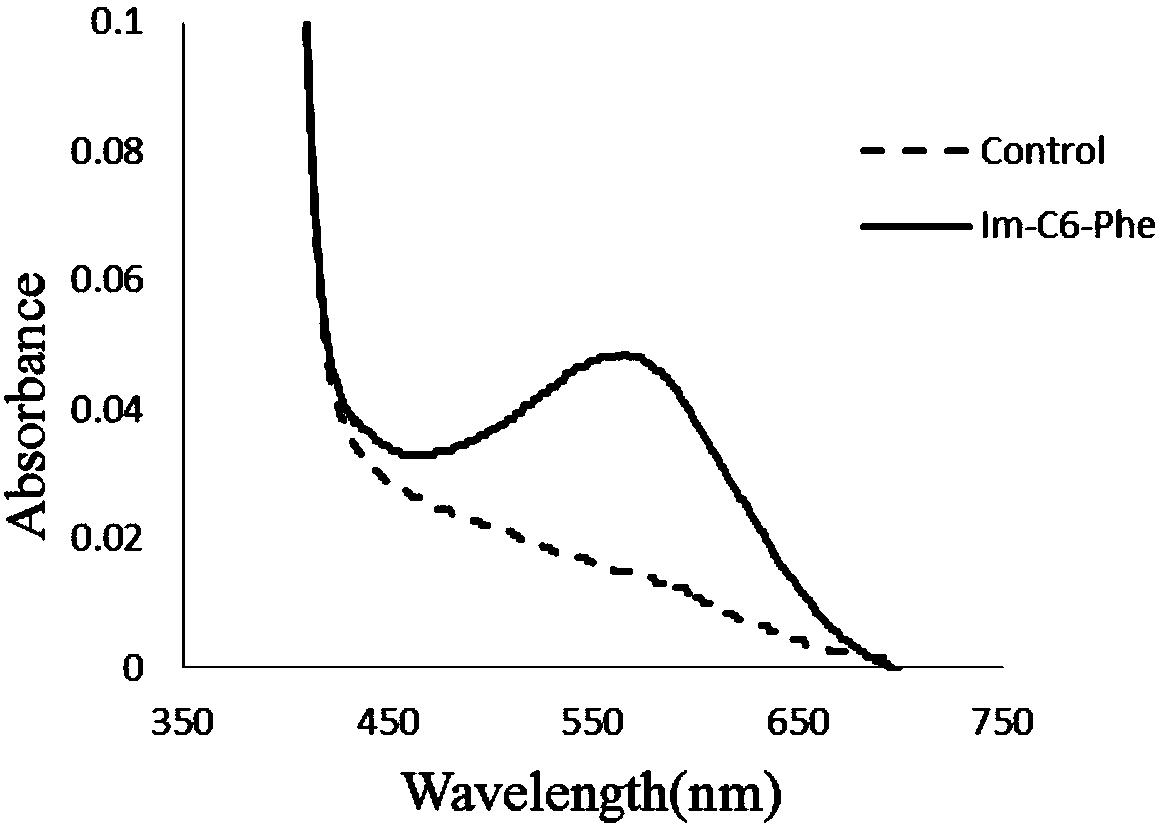
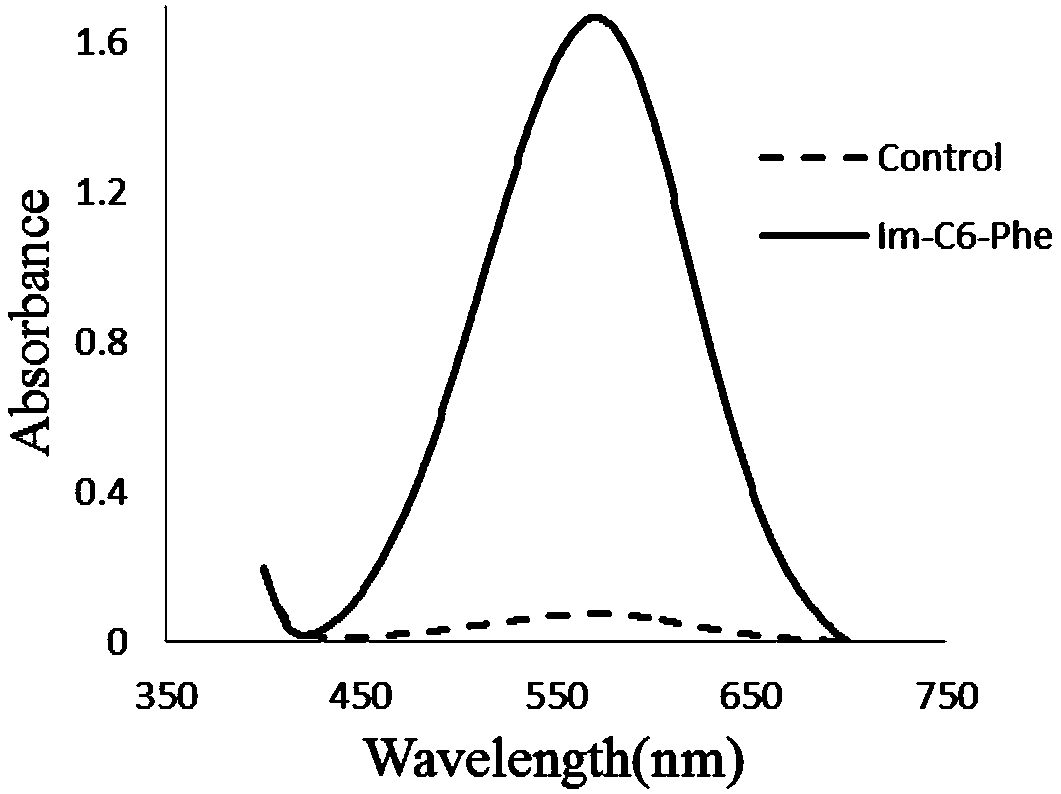
[0136] Depend on figure 1 and Table 1 can be seen join the pair The epoxidation reaction has a slight promoting effect.
Embodiment 2
[0138] F87A uses catalytic The epoxidation reaction:
[0139] The reaction system is as follows: 4mM (10% MeOH solubilization), 0.5 μM enzyme F87A (PatrickC.Cirino, Frances H.Arnold. Advanced Synthesis & Catalysis, 2010,344(9):932-937.), 0.5mM Small molecules, 20mMH 2 o 2 Add to PBS (100mM) with pH 8.0 in a final volume of 1mL, and react in a water bath at 25°C for 30min, then add 125μL of 4-(4-nitrobenzyl)pyridine (0.4g) in absolute ethanol (10mL), 80°C water bath for 30 min, then ice bath for 10 min, add 1 mL of acetone / triethylamine mixed solution (1:1). The 570nm absorption was measured with an ultraviolet spectrophotometer (Shimadzu Spectrophotometer UV-1800), and the catalytic reaction without small molecule compounds was used as a control (Table 2). The result of the reaction is as follows:
[0140] Table 2
[0141]
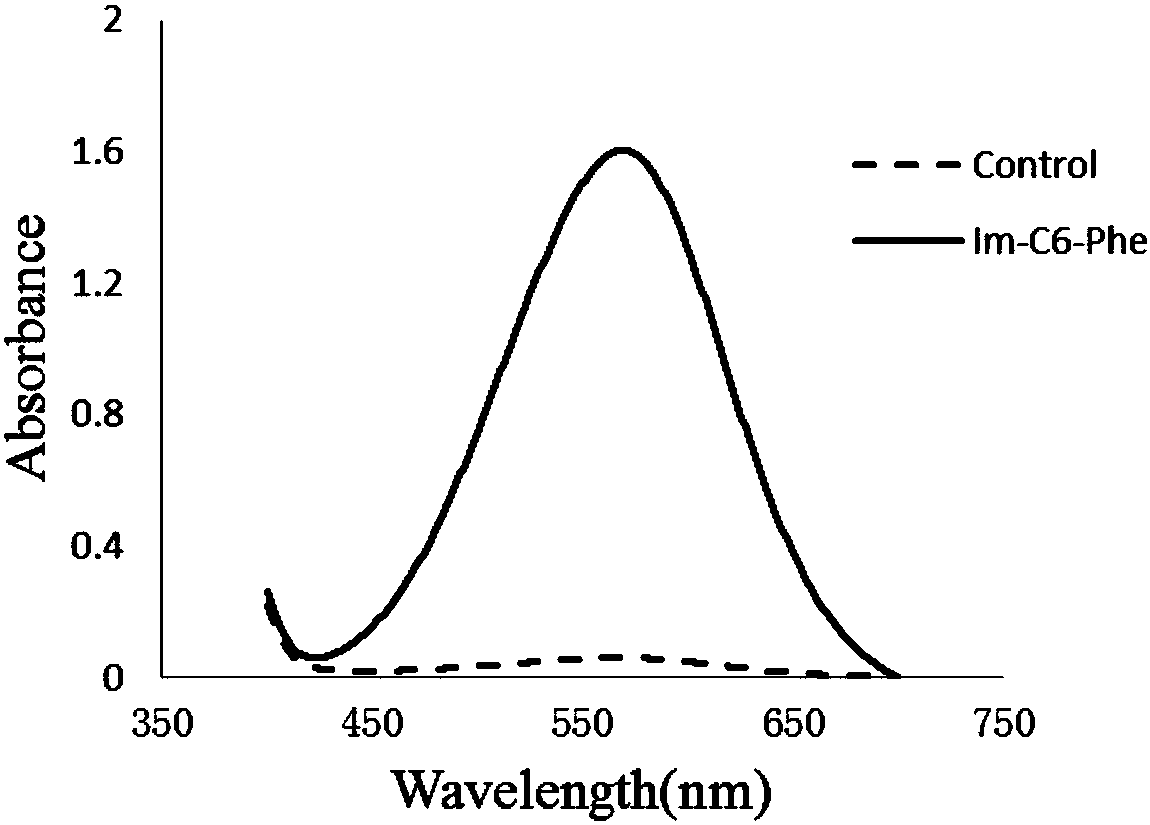
[0142] Depend on figure 2 and Table 2 can be seen join the pair The epoxidation reaction is greatly promoted.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com