Combination of novel vaccines against zika virus and DNA antibody constructs for use against zika virus
A technology of antibody and Zika, which is applied in the field of combination of Zika vaccine and composition, can solve the problems of high manufacturing and transportation costs of purified antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0328] The studies presented here demonstrate the generation of functional anti-Zika "DNA monoclonal antibodies" (DMAbs) by intramuscular electroporation of plasmid DNA. Codon-optimized variable region DNA sequences from anti-Zika monoclonal antibodies were synthesized onto human IgG1 constant domains. Plasmid DNA encoding the antibody was delivered to C3H mice. The study supports DMAb as an alternative to existing biological therapies.
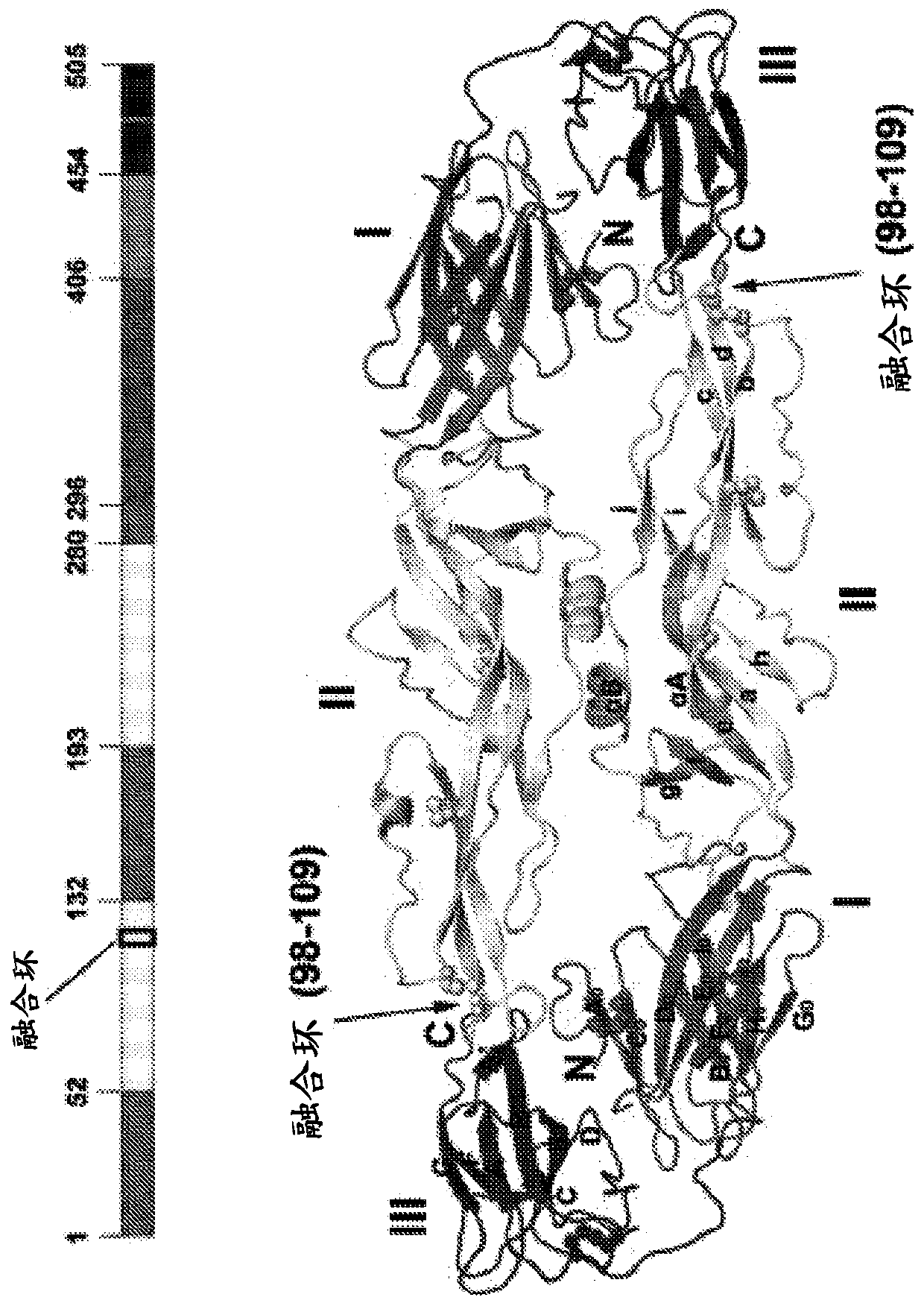
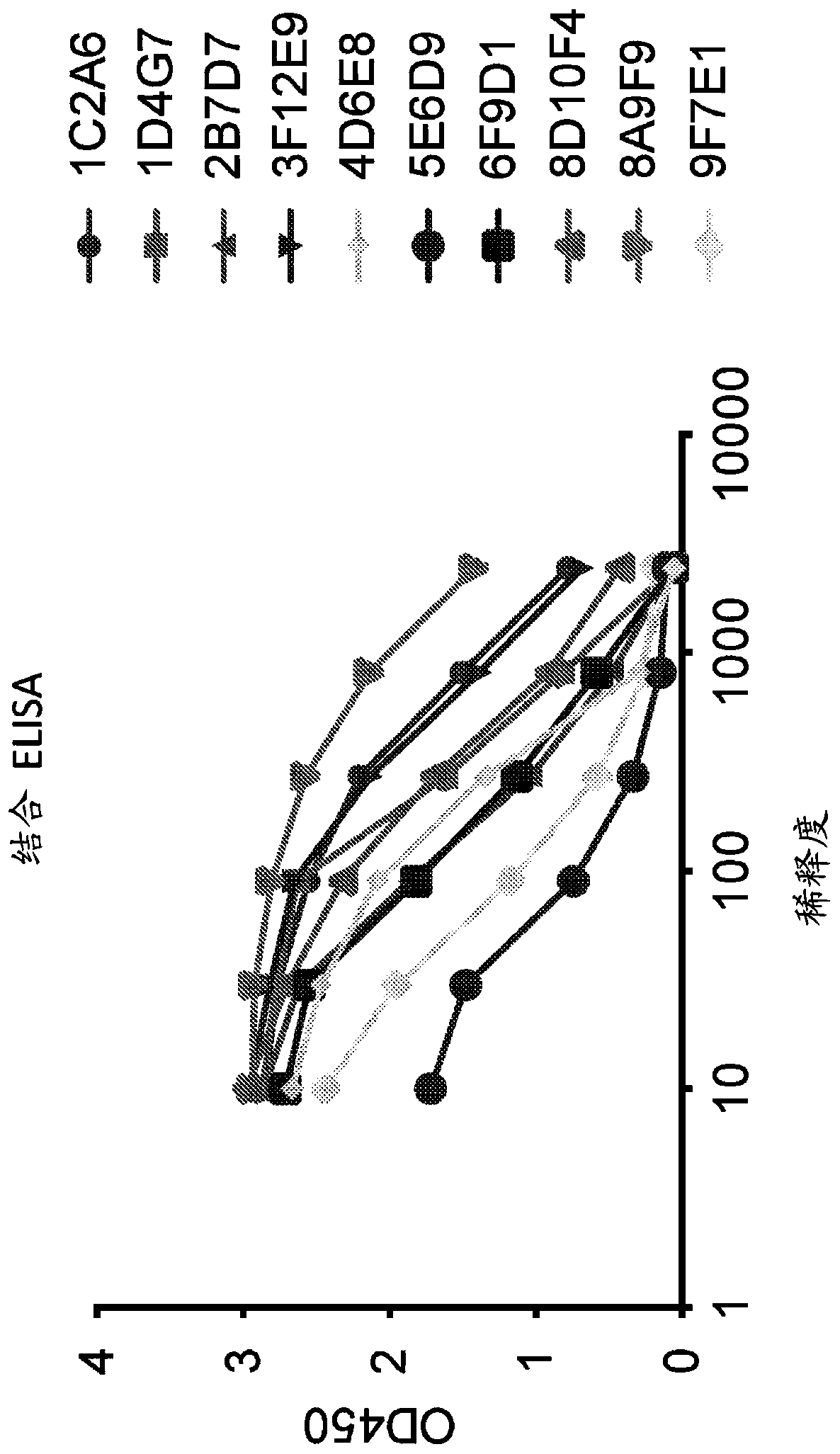
[0329] The ZIKV-Env (ZIKV-E) protein is a 505 amino acid protein with a fusion loop ( figure 1 ). Antibodies against the ZIKV-E protein were expressed in vivo by DNA monoclonal antibodies (dMAB) expressing heavy and light chains ( figure 2 ). ZIKV-Env-specific monoclonal antibodies 1C2A6, 1D4G7, 2B7D7, 3F12E9, 4D6E8, 5E6D9, 6F9D1, 9D10F4, 8A9F9 and 9F7E1 each bind ZIKV-Env in vitro ( Figure 3-4 ). monoclonal antibody in V H and V L chains showed varying degrees of sequence homology ( Figure 5-7 ). The large VH CDR3 of 1D4G7 is cl...
Embodiment 2
[0331] Zika vaccine approach
[0332] Such as Figure 13 As indicated, a Zika antigen expression construct was generated with the backbone indicated. The expression cassette was inserted behind the CMV promoter with a trailing polyadenylation tail. The box can include Figure 14 Coding sequences for antigens shown in , including prME, NS1 and capsid.
[0333] Phylogenetic analysis and vaccine design of Zika prME
[0334] Perform phylogenetic analysis, such as Figure 16 and 17 shown. The star shows the position of the consensus prME sequence SEQ ID NO:3. Show that the consensus sequence prME inserts according to Figure 18 cloning site in the expression vector.
[0335] Expressed proteins were characterized by Western blot analysis, as shown in Figures 20A and 20B, which show specific binding to anti-flavivirus antibodies.
[0336] The protein is then purified as Figure 21 A and 21B are shown.
[0337] mouse immunization
[0338] Animal-Balb / C mice (group of 8)...
Embodiment 3
[0355] Here we describe a novel synthetic DNA consensus-based vaccine targeting the precursor membrane + envelope proteins of Zika virus. Following confirmation of expression of the construct, mice and non-human primates were immunized by electroporation, showing the induction of cellular and humoral immunity with neutralizing activity in the vaccinated animals. in IFN-α / βR - / - In mice, single or double injection immunization was 100% protective against weight loss or death in this lethal challenge model. This is the first Zika virus vaccine approved for human trials.
[0356] Materials and methods are now described.
[0357] Cells, Viruses and Animals
[0358] Human embryonic kidney (HEK) 293T (American Type Culture Collection (ATCC) #CRL-N268, Manassas, VA) and Vero CCL-81 (ATCC #CCL-81 ) cells were maintained at supplemented with 10% Fetal bovine serum (FBS) and 1% penicillin and streptomycin in Dulbecco's modified Eagle's medium (DMEM; Gibco-Invitrogen), and passaged...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap