Improved methods of assessing gfap status in patient samples
A state, biological sample technology, applied in the direction of chemical instruments and methods, biochemical equipment and methods, instruments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
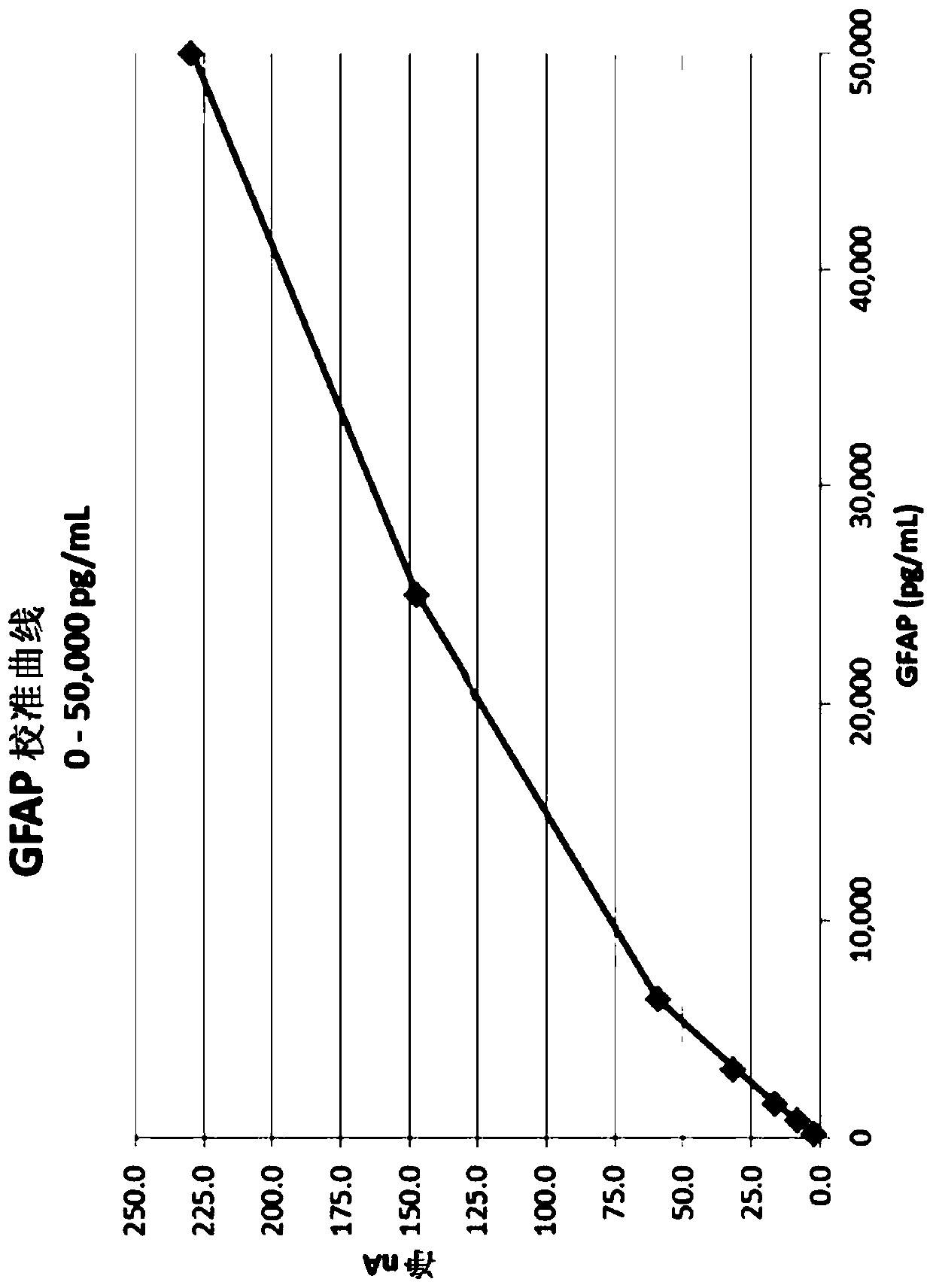
[0375] GFAP assay
[0376] Screening of antibodies employs a target assay format (i-STAT). Select antibody pairs that generate a signal in the assay. Initial selection criteria were based on several factors, including the use of paired antibody detection signals when screening with low calibrator concentrations. Test monoclonal antibody pairs, such as Antibody A as a capture mAb and Antibody B as a detection mAb. Antibody A and Antibody B are exemplary anti-GFAP antibodies developed in-house at Abbott Laboratories (Abbott Science Park, IL). Antibody A and Antibody B both bind the epitope in the same GFAP breakdown product (BDP). When used in combination, the combination of antibodies produces a synergistic effect and a stronger signal. This data is generated by purchasing short overlapping peptide sequences and determining which peptides the antibody binds to in a 96-well plate format. The GFAP assay design is evaluated against key performance attributes. Cartridge con...
Embodiment 2
[0413] potential assay interference
[0414] An evaluation of potential interferences and cross-reactants for the GFAP assay is shown in Table 12. Briefly, the interferor was spiked into an aliquot of GFAP strip at a target concentration of 150-200 pg / mL. Interference testing concentration based on CLSI EP7-A2 guidelines (Clinical and Laboratory Standards Institute.Interference Testing in ClinicalChemistry; Approved Guideline-Second Edition.CLSI document EP7-A2[ISBN 1-56238-584-4].Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2005.). Testing for potential cross-reactants was performed at a concentration level of 500 ng / mL. The acceptance criterion was interference <10%.
[0415] Specifically, GFAP assays were evaluated for potential endogenous interference. Potential interfering substances are prepared in selected buffer solutions / solvents and then added to test samples containing target analytes. A cont...
Embodiment 3
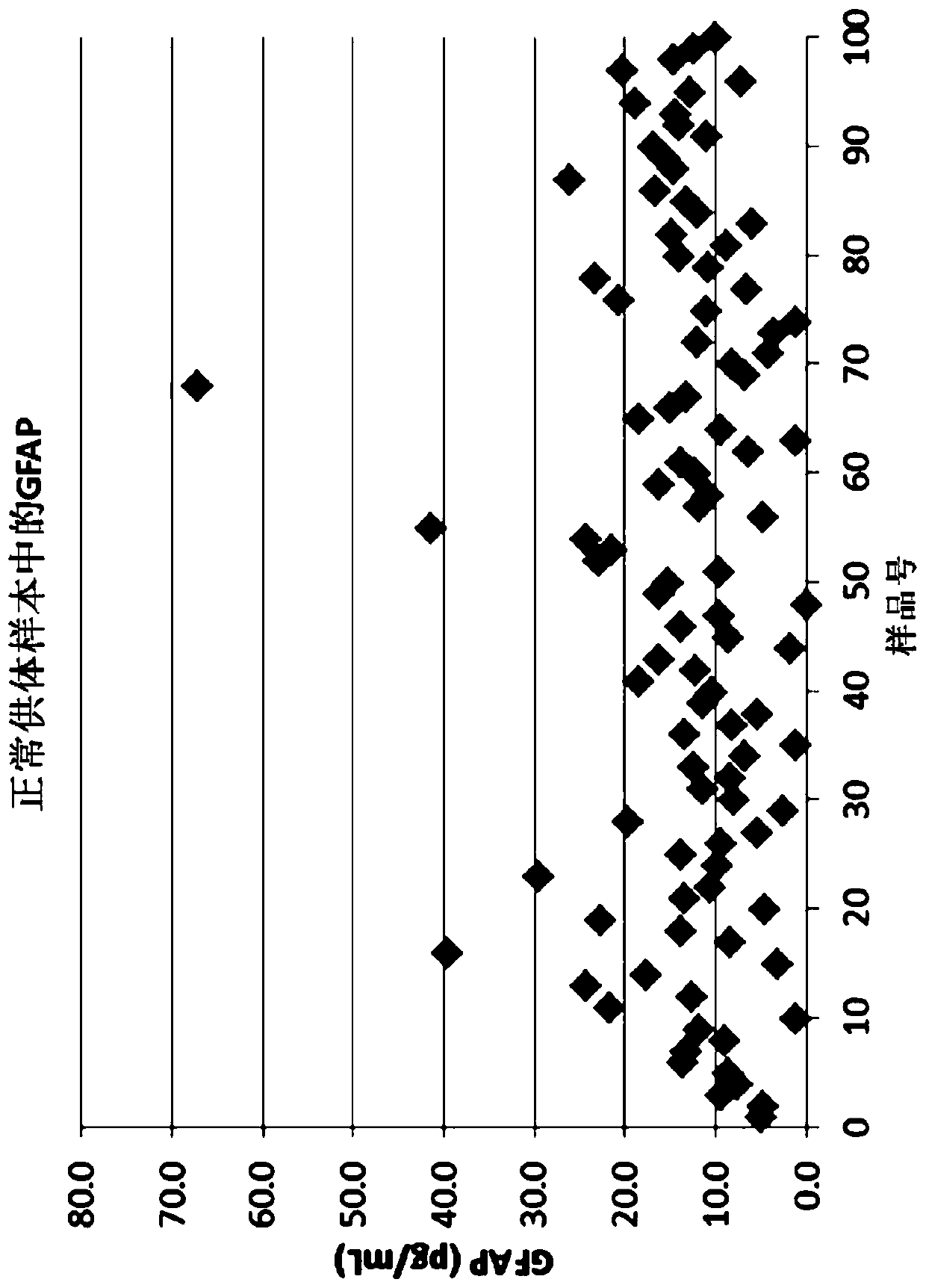
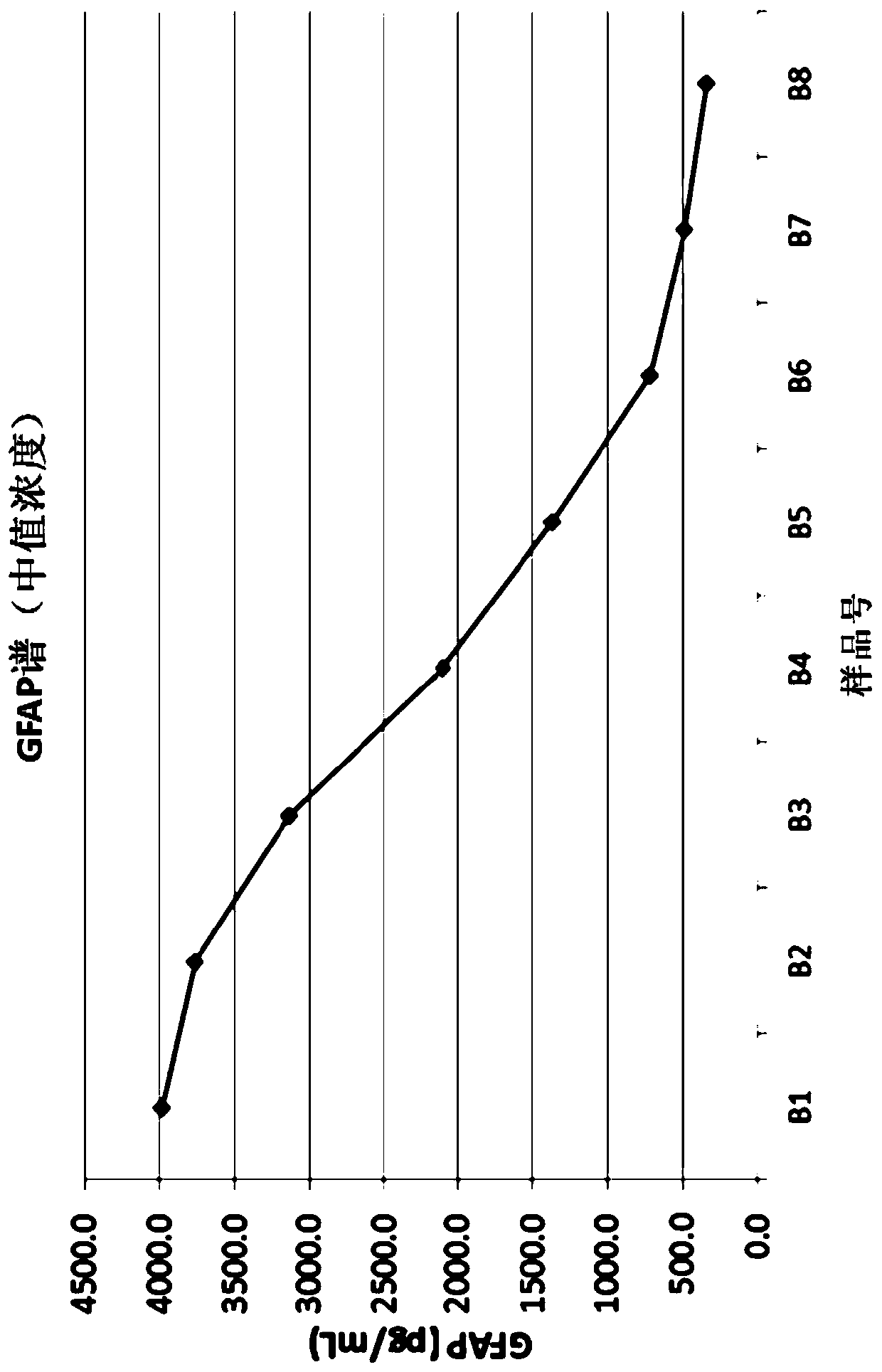
[0427] TBI Population Study
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com