Synthetic immune receptors and methods of use thereof
An immunoreceptor and sequence technology, applied in medical preparations containing active ingredients, fusion polypeptides, antibody medical components, etc., can solve the problems of apoptosis, immunological depletion, excessive cytokine release, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
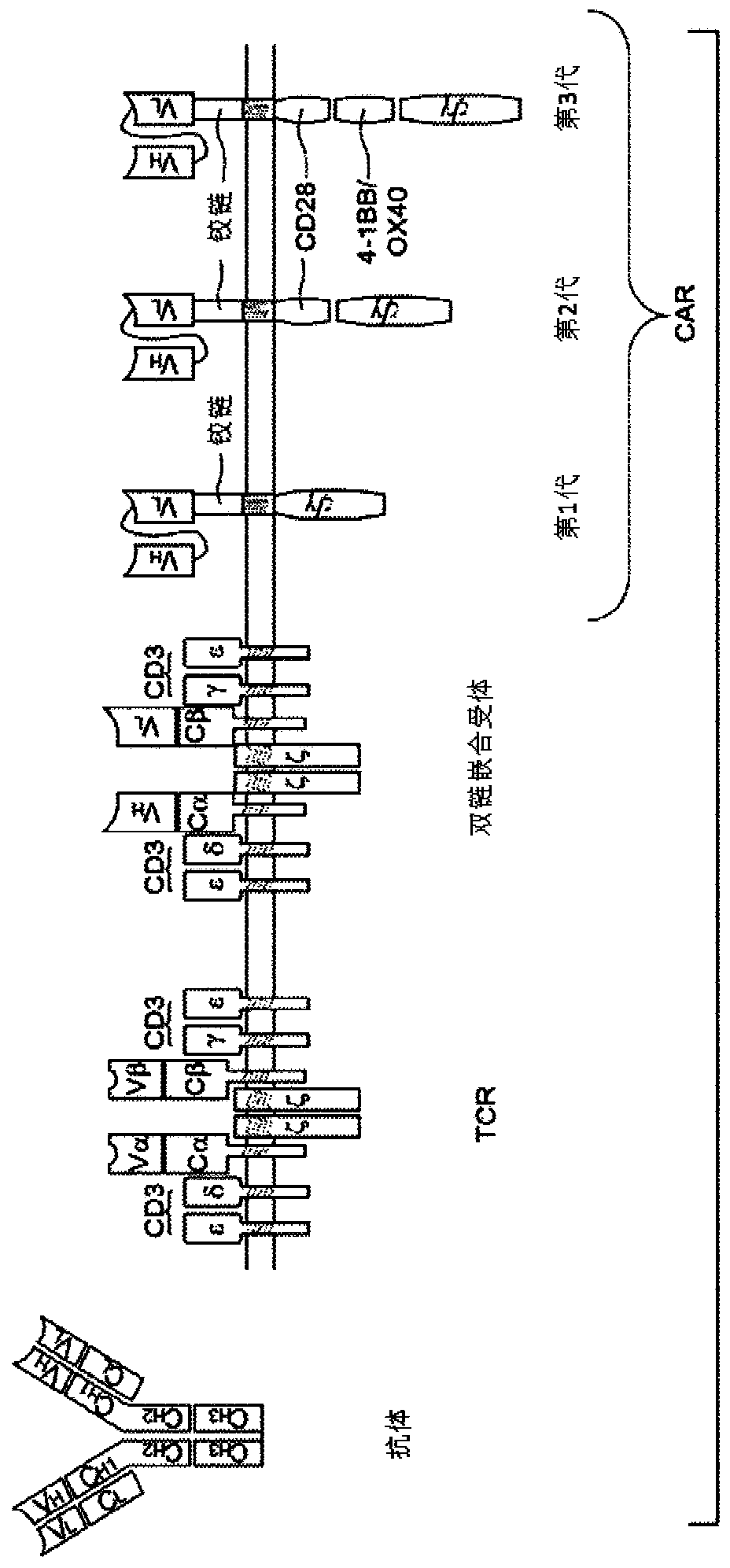
[0569] The activity of SIRs can be tested by several in vitro and in vivo assays described herein and below. A general scheme for generating, selecting and using suitable SIRs is provided below.
[0570] Identification of targets for SIR generation Suitable targets for designing SIRs are selected based on searches of libraries or gene expression databases. In general, suitable targets of SIRs show higher expression on disease-causing or disease-associated cells compared to normal healthy cells.
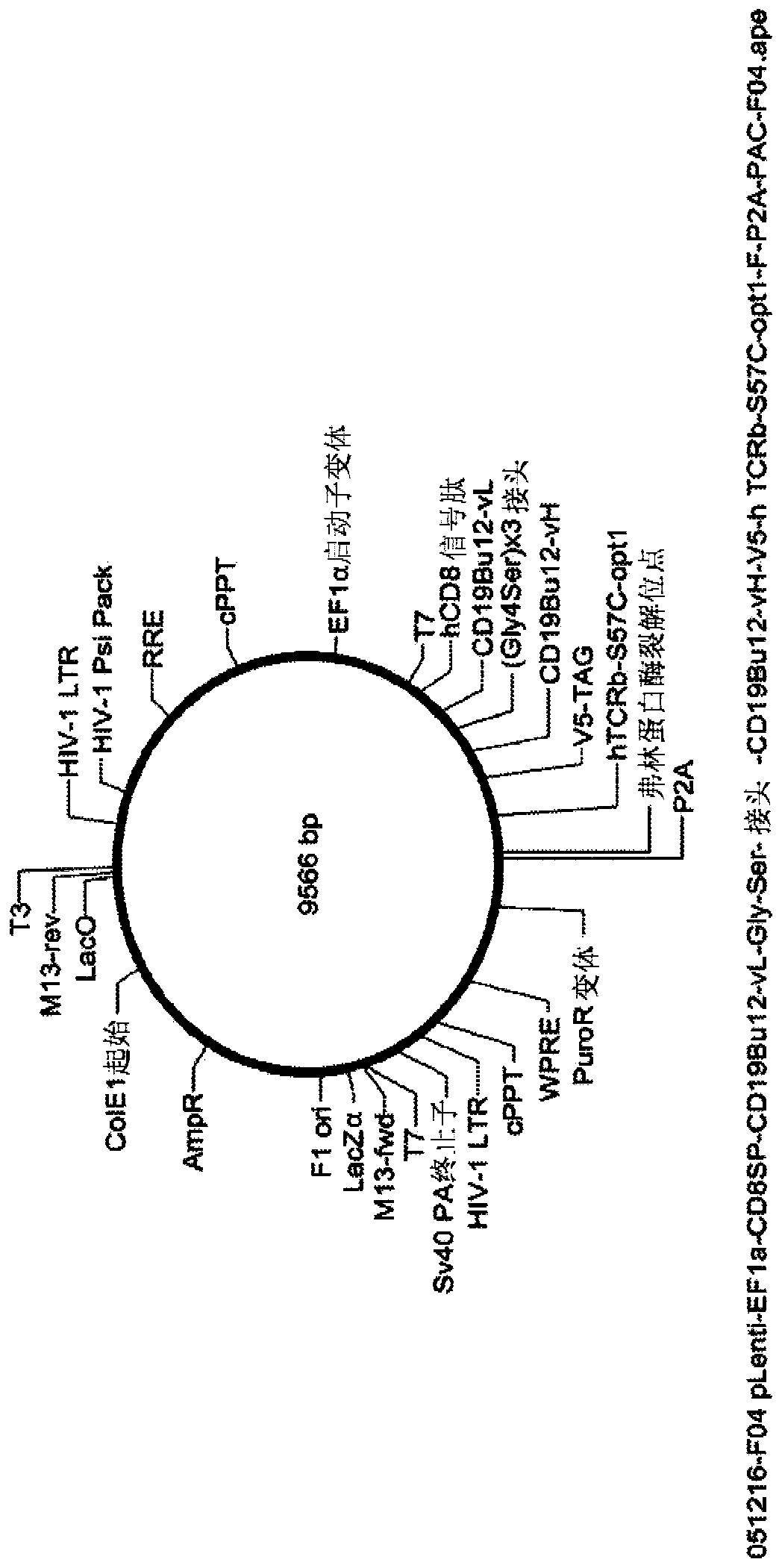
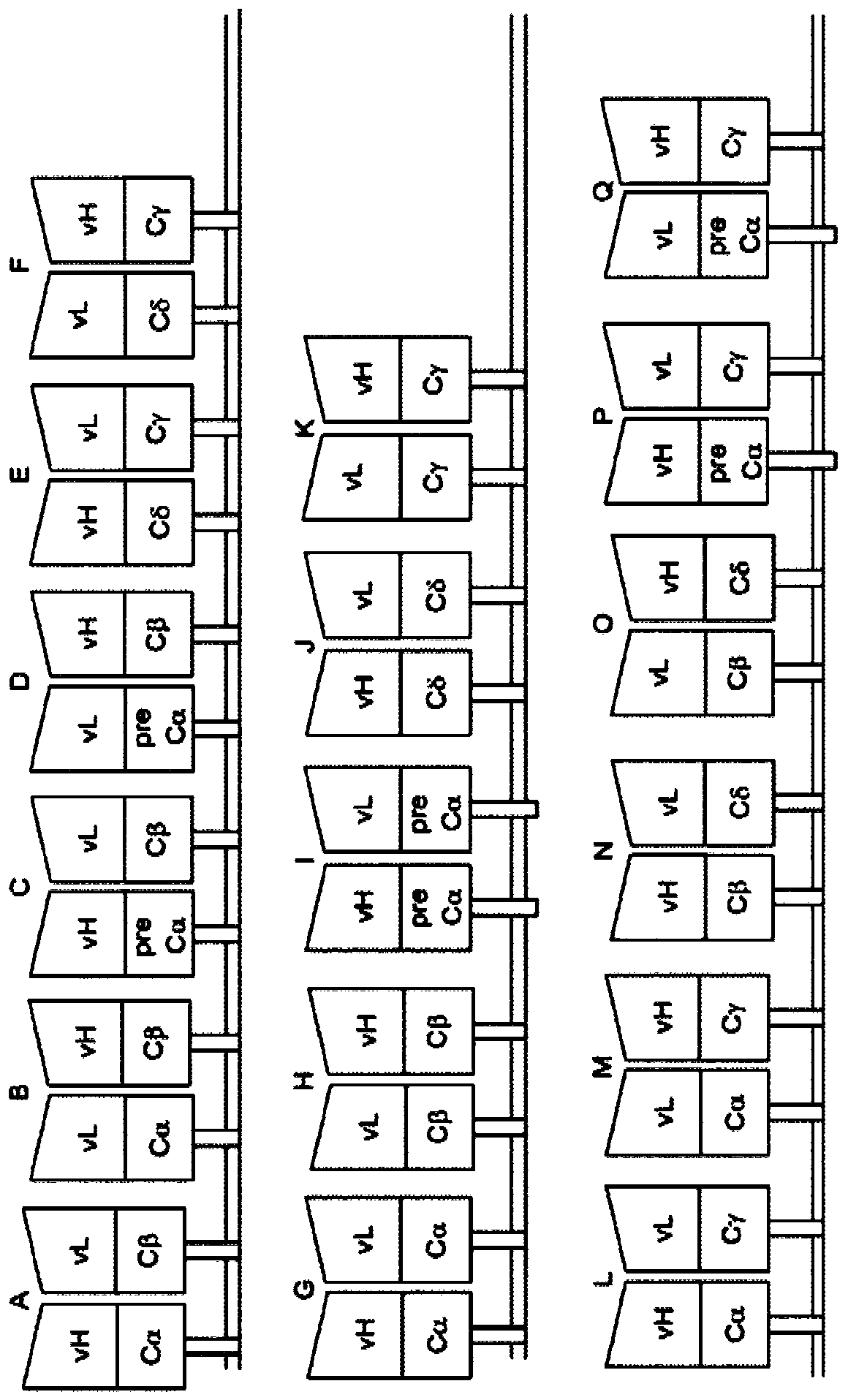
[0571] Generation of SIRs. Once the candidate target antigens of the SIRs are identified, the antigen-binding domains of the SIRs are designed based on the information available in the library. In general, the antigen binding domains of SIRs are typically based on antibodies, antibody fragments, scFV, or camelid vHH domains. The sequences of antibodies, camelid vHH domains, and variable chains of the heavy (vH) and light (vL) chains of various receptors and ligands can be obtained b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com