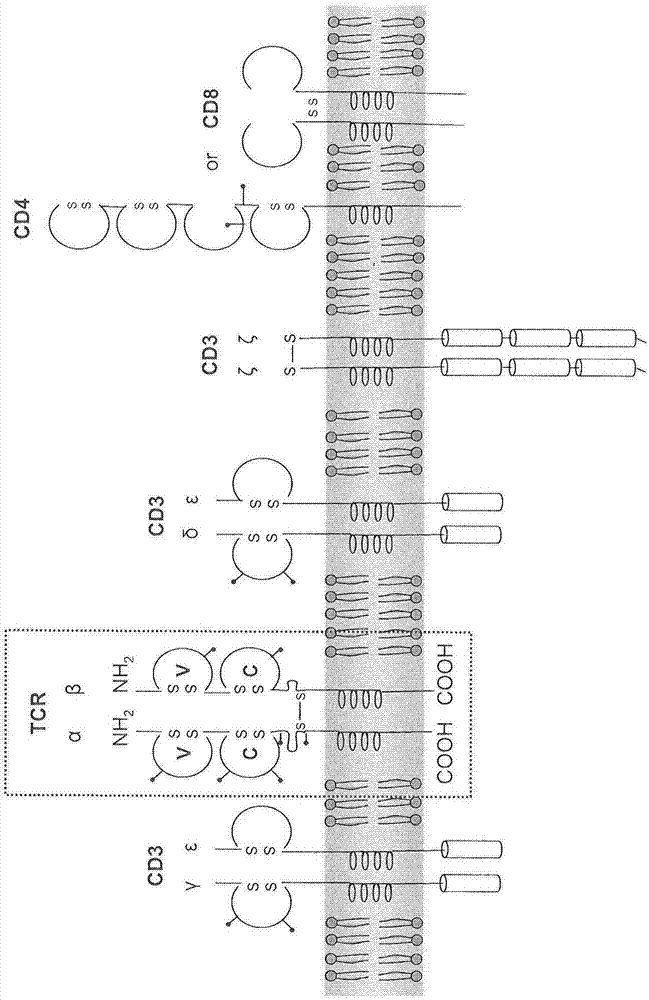
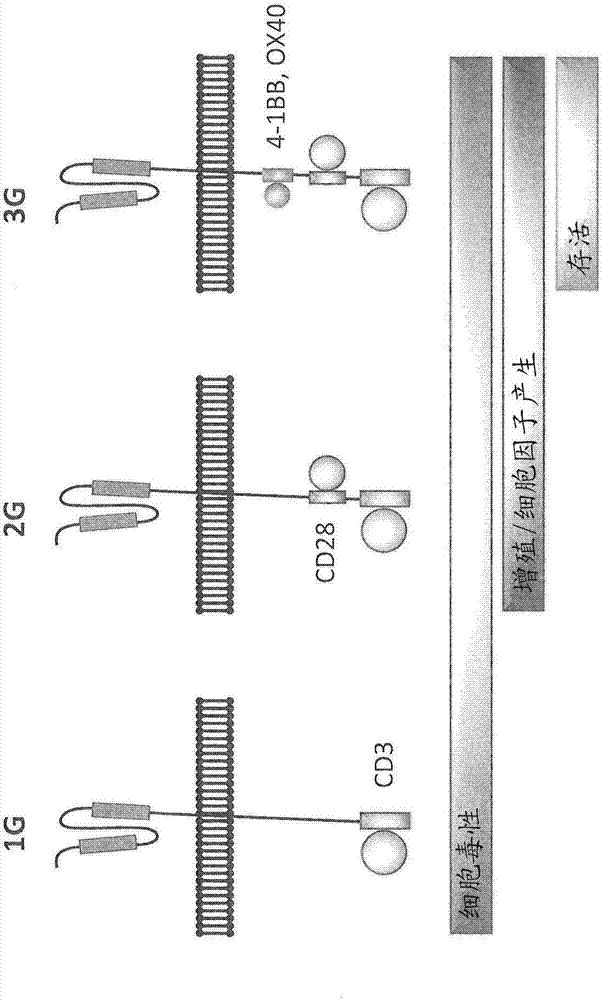
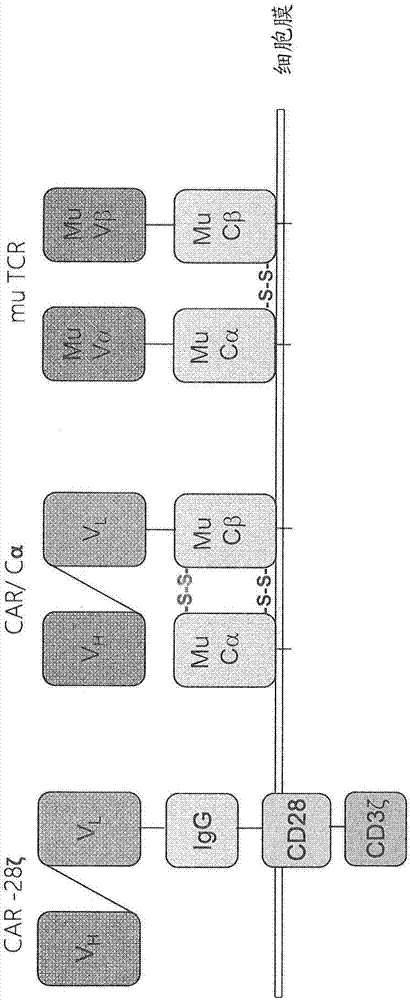
Claudin-18.2-specific immunoreceptors and T cell epitopes
A cell and cell receptor technology, applied in receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, for targeting specific cell fusion, etc. Able to solve problems that have not been described, not known, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0435] Example 1: Materials and methods
[0436] Cell Lines and Reagents
[0437] The human chronic myeloid leukemia cell line K562 (Lozzio, C.B. & Lozzio, B.B (1975), Blood 45, 321-334) stably transfected with HLA-A*0201 and used for TCR validation assays was cultured under standard conditions (Britten, C.M. et al. (2002), J. Immunol. Methods 259, 95-110) (referred to eg as K562-A2). The primary human neonatal foreskin fibroblast cell line CCD-1079Sk (ATCC No. CRL-2097) was cultured according to the manufacturer's instructions.
[0438] Peripheral blood mononuclear cells (PBMC), monocytes and dendritic cells (DC)
[0439] PBMCs were isolated from buffy coats by Ficoll-Hypaque (Amersham Biosciences, Uppsala, Sweden) density gradient centrifugation. HLA alleles were determined by PCR standard methods. Monocytes were enriched with anti-CD14 microbeads (MiltenyiBiotech. Bergisch-Gladbach, Germany). Immature DCs (iDCs) were obtained by differentiating monocytes in medium supp...
Embodiment 2
[0481] Example 2: Isolation of a high-affinity HLA-A*02-restricted murine TCR specific for claudin 18.2
[0482] We verified the immunogenic potential of CLDN18.2 in A2 / DR1 mice by repeated intranodal immunizations with IVT-RNA encoding amino acids 1-80 of CLDN18.2. The human CLDN18 gene has 2 alternative first exons, resulting in 2 protein isoforms (CLDN18.1 and CLDN18.2) that differ by 69 amino acids at the N-terminus ( Figure 4 A). Since CLDN18.1 is also expressed in normal tissues, especially the lung, we only used the N-terminal part of CLDN18.2 in order to exclusively induce CLDN18.2-specific T cell reactivity. We used splenocytes from these mice for isolation of CLDN18.2-specific T cells and subsequent cloning of the corresponding TCR genes. Splenocytes from immunized mice that successfully induced CLDN18.2-specific T cells were analyzed and analyzed ex vivo for reactivity to predicted HLA-A*02-binding CLDN18.2 peptides by an IFNγ-ELISPOT assay ( Figure 5 ).
[04...
Embodiment 3
[0487] Example 3: Generation and in vitro validation of claudin-18.2 specific CAR
[0488] We produced a second-generation CAR targeting CLDN18.2, which contains the signaling and co-stimulatory portions of CD3ζ and CD28, respectively. Deletion of the lck-binding moiety in the intracellular structure of CD28 abolishes IL2 secretion upon CAR engagement to prevent induction of regulatory T cells (Kofler D.M. et al., (2011) Molecular Therapy 19(4), 760–767). Modification of the IgG1 Fc "spacer region" domain in the extracellular part of the CAR avoids "off-target" activation and unintended initiation of innate immune responses (Hombach A. et al., (2010) Gene Therapy 17, 1206–1213 ).
[0489] To analyze the specific lysis of CLDN18.2-expressing target cells by CLDN18.2-CAR T cells, a luciferase-based cytotoxicity assay was performed. CD8+ T cells were preactivated and CD8+ T cells were transfected with IVT-RNA encoding CLDN18.2-CAR or CLDN6-specific CAR (as control). Surface ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com