Methods of immune modulation against foreign and/or auto antigens
An antigen and immune checkpoint technology, applied in the field of immune regulation against foreign antigens and/or self-antigens, can solve the problems of lack of tumor antigen-reactive clones, low response rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
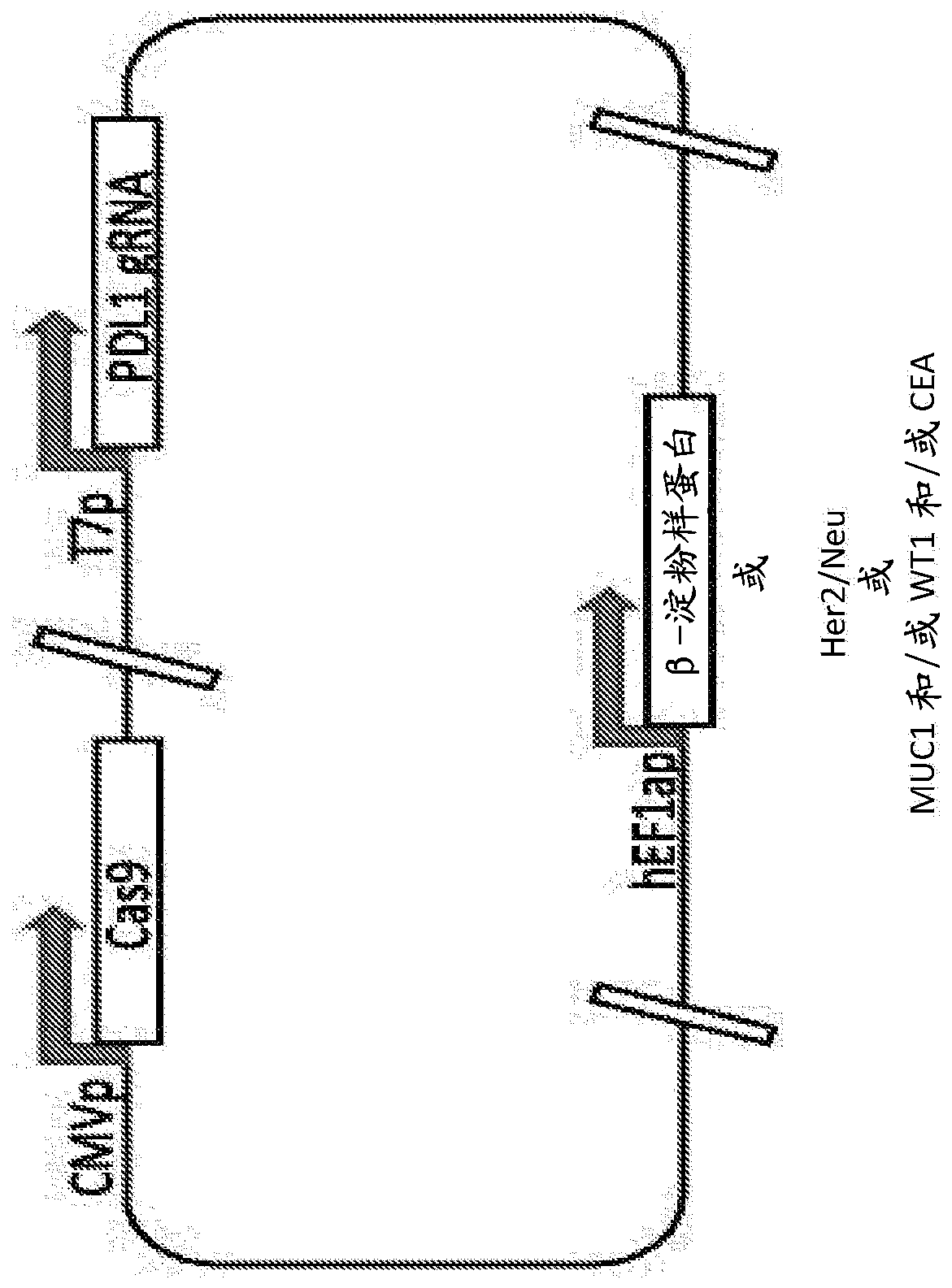
[0235] Utilize one or more guide RNAs targeting human PDL1, CRISPR-Cas9 protein, and antigenic protein β-amyloid protein with the sequences in Table 2 below to design and construct plasmids:
[0236] Table 2.
[0237]
[0238]
[0239] The CRISPR-Cas9 protein is expressed under the control of the CMV promoter, the guide RNA is expressed by the T7 promoter, and the β-amyloid protein is expressed under the control of the hEFla promoter, such as figure 1 drawn in. although figure 1 Single plasmid constructs are shown, but expression of these elements in multiple DNA plasmids is also within the scope of the invention. The plasmid is delivered to a patient suffering from, for example, Alzheimer's disease. Current vectors can be chemically synthesized based on publicly available sequence information.
Embodiment 2
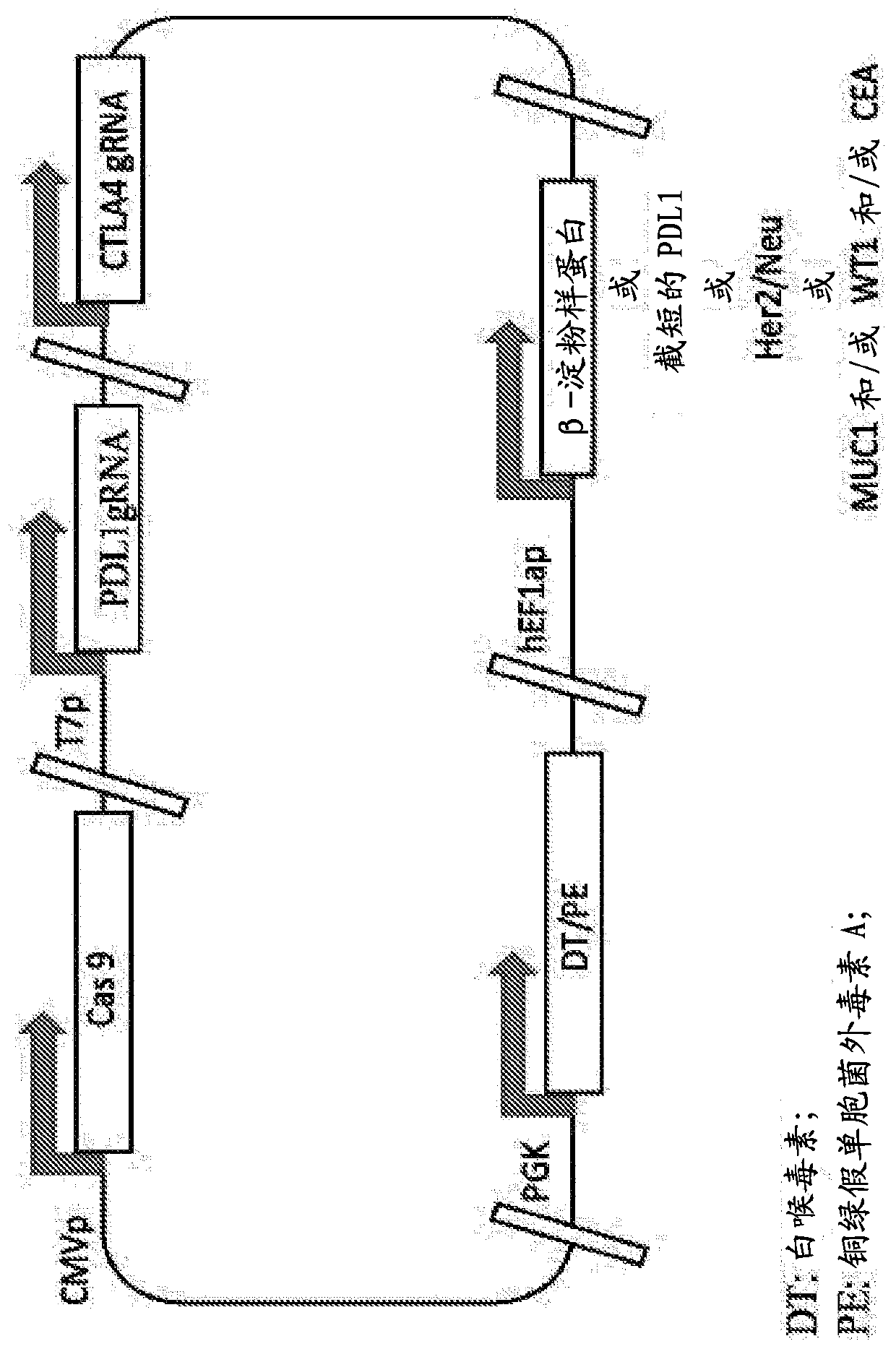
[0241] Single plasmids were constructed as described in Example 1 above using guide RNA targeting PDL1 and / or CTLA4 (Table 1 above), CRISPR-Cas9 protein, and Her2 / Neu. figure 2 Depicted is a single plasmid in which the CRISPR-Cas9 protein is expressed from the CMV promoter, the guide RNA is expressed from the T7 promoter, and Her2 / Neu is expressed from the h-EFla promoter. Such compositions can be used to treat diseases such as, but not limited to, breast cancer or other Her2 / Neu expressing cancers.
Embodiment 3
[0243] Single plasmids were constructed as described in Example 1 above using guide RNA targeting PDL1 and / or CTLA4 (Table 1 above), CRISPR-Cas9 protein, and Her2 / Neu. figure 2 Depicted is a single plasmid in which the CRISPR-Cas9 protein is expressed from the CMV promoter, the PDL1 guide RNA is expressed from the T7 promoter, the CTLA4 gRNA is expressed under the control of the promoter, and Her2 / Neu is expressed from the h-EFla promoter.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap