A kind of glucose oxidase mutant and its carrier and application
A technology of glucose oxidase and mutants, applied in the field of glucose oxidase mutants and their carriers and applications, can solve the problems of high production cost, limited industrial application, low specific activity of GOD, etc., and achieve the effect of high relative enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1, optimization and vector construction of Aspergillus niger (Aspergillus niger) glucose oxidase (GOD) gene
[0037] The glucose oxidase (GOD) gene (Genebank: FJ979866.1) of Aspergillus niger Aspergillus niger GIM 3.452 (CICC 2377), its nucleotide sequence is shown in SEQ ID NO: 2, and its amino acid sequence is shown in SEQ ID NO: 1 Show.
[0038]Through a large number of previous studies, it was found that the expression level of Aspergillus niger expression engineering bacteria was low, the operation was complicated, and it was difficult to perform high-throughput screening of mutations. At the same time, it was found that the glucose oxidase expressed in the Escherichia coli expression system had no activity and could not be used for mutation screening. Pichia pastoris is a mature exogenous protein expression platform for eukaryotic microorganisms, which has the following characteristics: rapid growth and cheap culture; simple and easy to operate, conven...
Embodiment 2
[0039] Embodiment 2, glucose oxidase gene site-directed mutation
[0040] Using the above pGAPzαA-GOX as a template, introduce site-directed mutation and transform Pichia pastoris X33
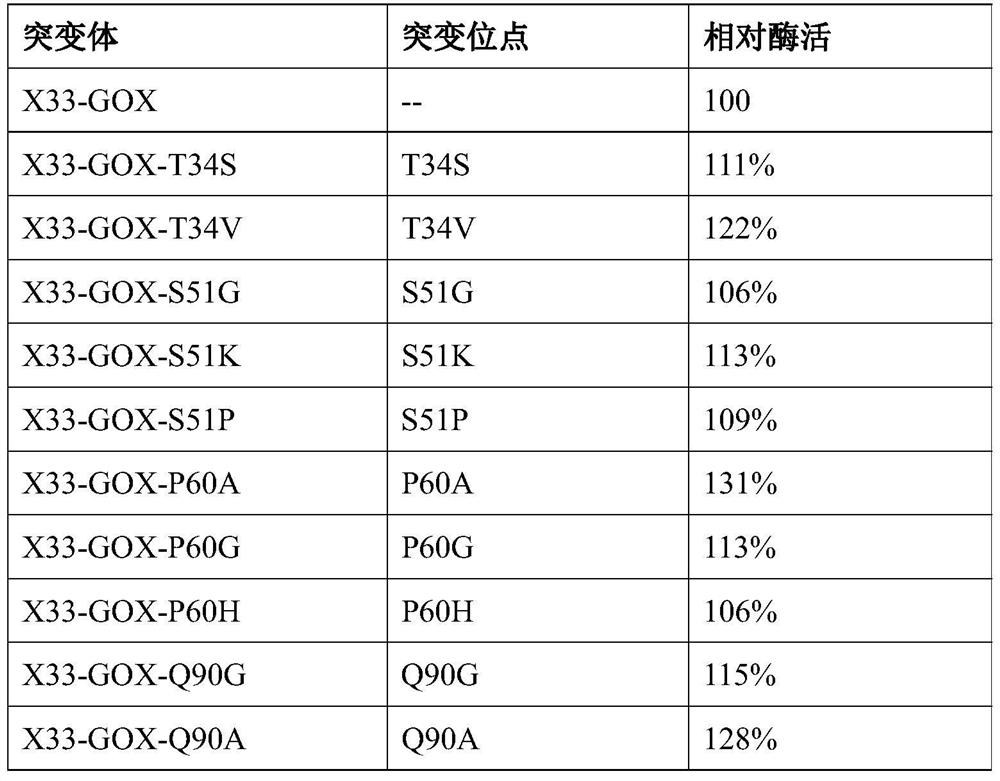
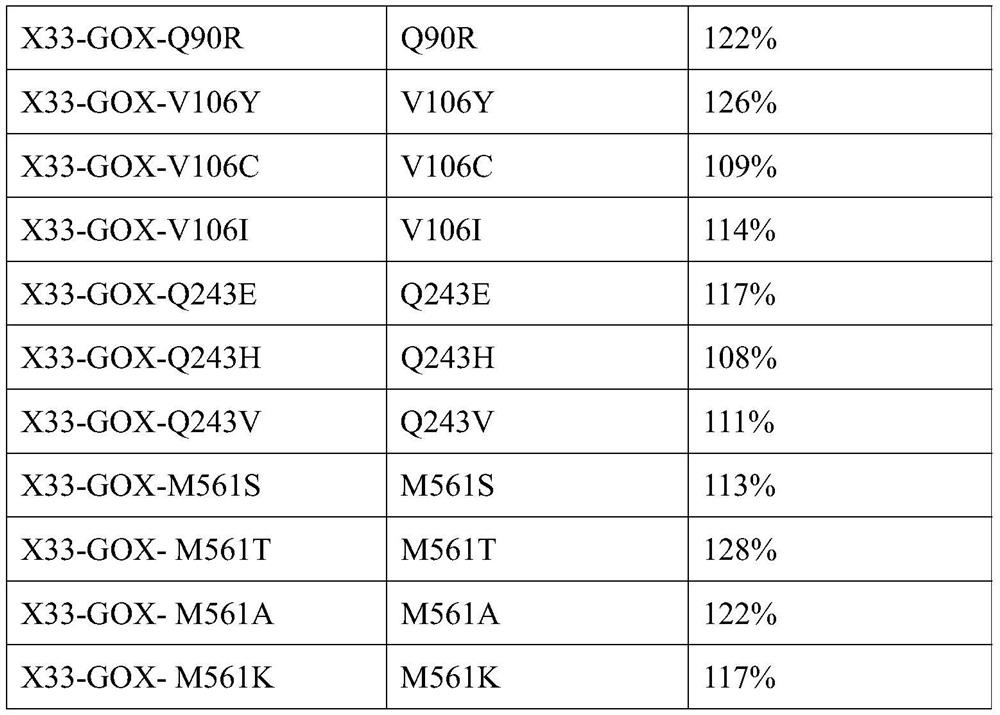
[0041] According to the three-dimensional structure information of glucose oxidase, after a lot of research, it is believed that the 34th, 51st, 60th, 90th, 106th, 243rd, 420th, and 561st amino acids in the amino acid sequence have important effects on the enzymatic activity of glucose oxidase, and saturation mutations are carried out on the above sites , Pick the yeast recombinant transformants obtained by the site saturation mutation one by one to a 24-well plate, add 1 mL of medium containing BMGY to each well, culture at 30°C, 220 rpm for about 24 hours, and centrifuge to remove the supernatant. Then add 1.6mL BMMY medium respectively for induction culture. After culturing for 24 hours, the supernatant was collected by centrifugation, and 200 μL of the above supernatant was taken out to a ...
Embodiment 3
[0049] Embodiment 3, glucose oxidase gene combination mutation
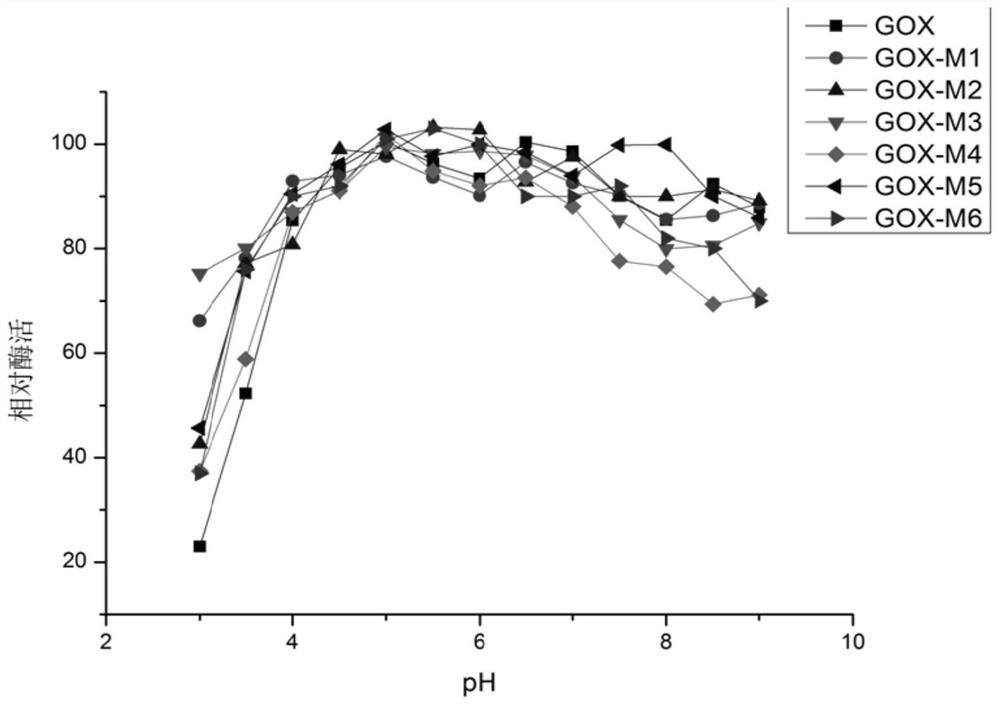
[0050] Effective mutation T34S, V; S51G, K, P; P60A, G, H; Q90G, A, R; V106Y, C, I; Q243E, H, V; , A are combined separately. Finally, six combined mutations with significantly improved enzyme activity were obtained through experiments and named GOX-M1, GOX-M2, GOX-M3, GOX-M4, GOX-M5, and GOX-M6, respectively.
[0051] The mutation sites included in GOX-M1 are: T34S, S51K, P60A, Q90A, V106Y, Q243E, M561S, its amino acid sequence is shown in SEQ ID NO: 4, and its corresponding nucleotide sequence is shown in SEQ ID NO: 10 Show.
[0052] The mutation sites included in GOX-M2 are: T34V, S51P, P60A, Q90R, V106Y, Q243H, M561T, its amino acid sequence is shown in SEQ ID NO: 5, and its corresponding nucleotide sequence is shown in SEQ ID NO: 11 Show.
[0053] The mutation sites included in GOX-M3 are: T34V, S51P, P60A, Q90R, V106I, Q243E, M561S, its amino acid sequence is shown in SEQ ID NO: 6, and its corresponding...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com