Chimeric antigen receptor comprising costimulatory receptor and application thereof
A chimeric antigen receptor and co-stimulation technology, which can be used in medical preparations containing active ingredients, fusion polypeptides, anti-tumor drugs, etc. It can solve the problems of immune side effects, poor efficacy, and impact on CAR-T.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-20B
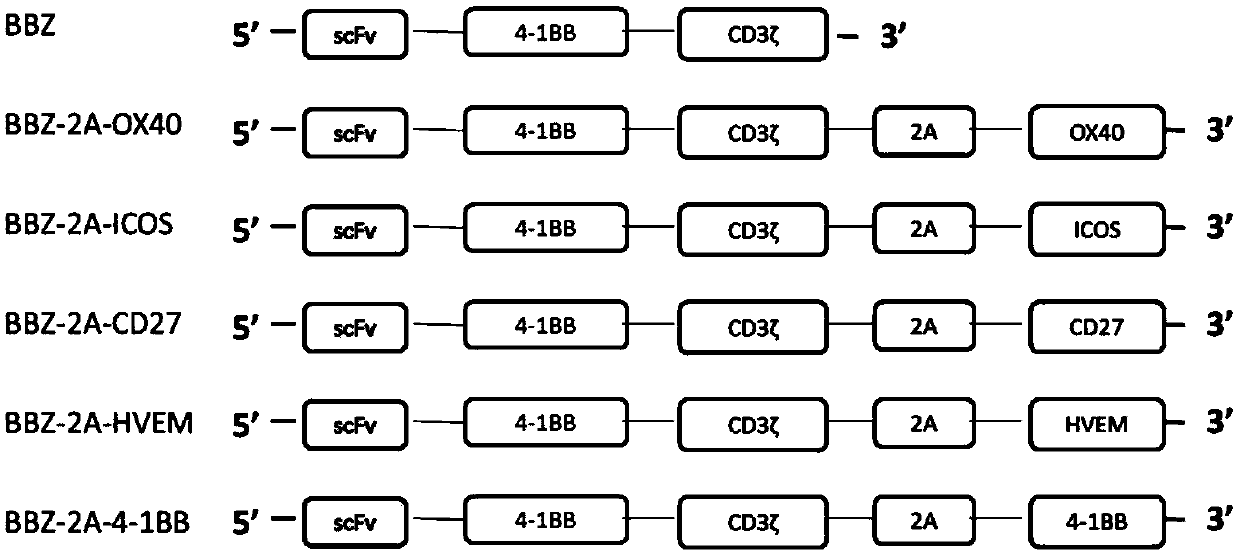
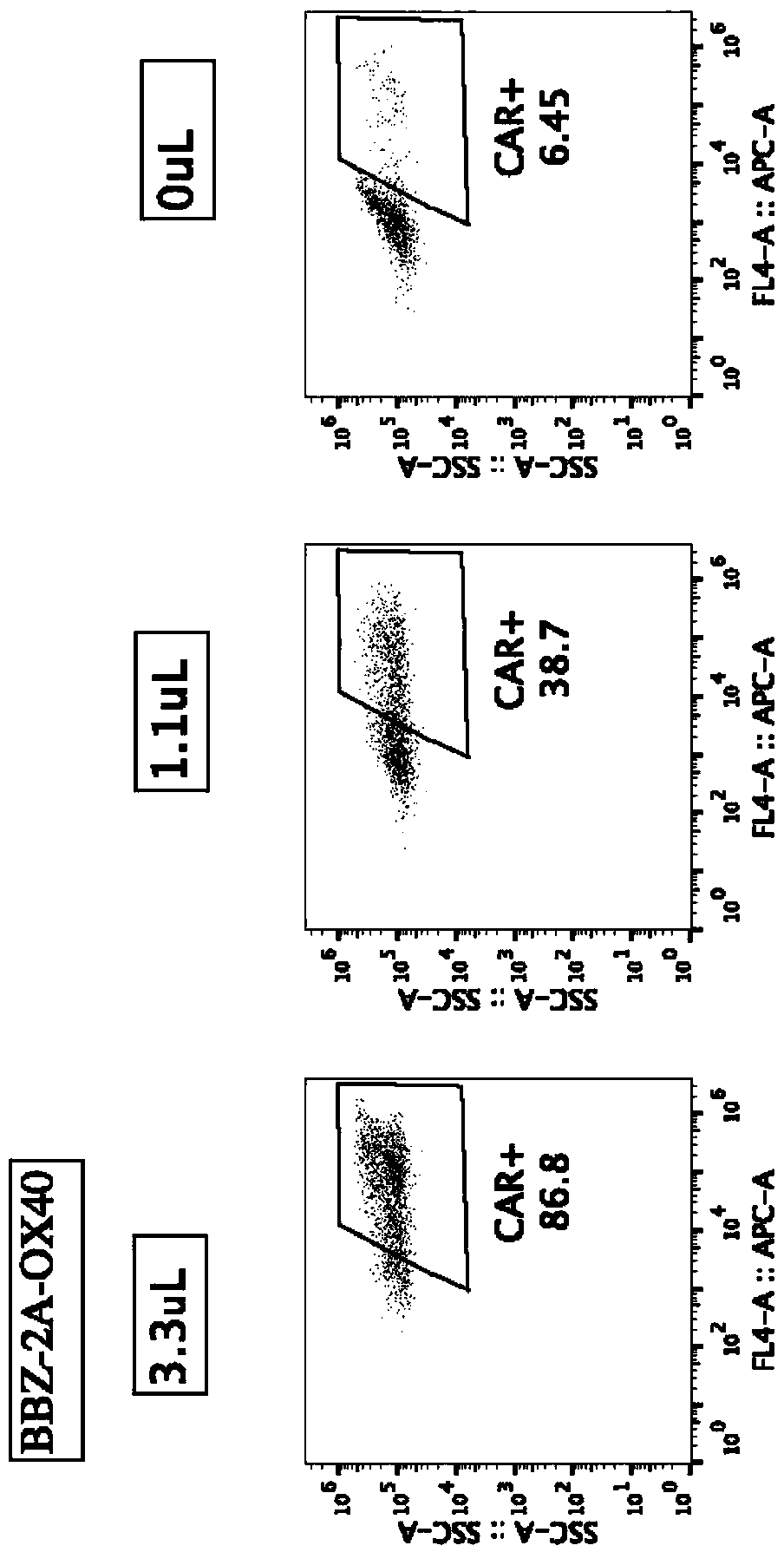
[0074] Example 1-20 Preparation of BBZ-2A-OX40CAR-T cells
[0075] The preparation of 20BBZ-2A-OX40CAR-T cells described in this example includes the following steps:
[0076] 1. Construction of lentiviral vector pCDH-MSCVEF-20BBZ-2A-OX40 and virus production
[0077] Add 2A (SEQ ID No.7) sequence to the middle of scFv-antihCD20-20BBZ (SEQ ID No.1) and OX40 (SEQ ID No.2) through overlap PCR, and add EcoRI and SalI restriction sites at both ends to clone pCDH - MSCVEF vector. The clones with correct sequencing were extracted without endotoxin, and co-transfected with lentiviral packaging plasmids (VSV-g, pMD Gag / Pol, RSV-REV) at 293X. After 48 and 72 hours, the supernatant was collected, filtered at 0.45uM, and used Beckman ultracentrifuge and SW28 rotor, 25000RPM centrifugation for 2 hours to concentrate the virus, which is pCDH-MSCVEF-20BBZ-2A-OX40 virus (abbreviated as 20BBZ-2A-OX40 virus), for subsequent CAR-T cell production. Produce contrast pCDH-MSCVEF-20BBZ virus (ab...
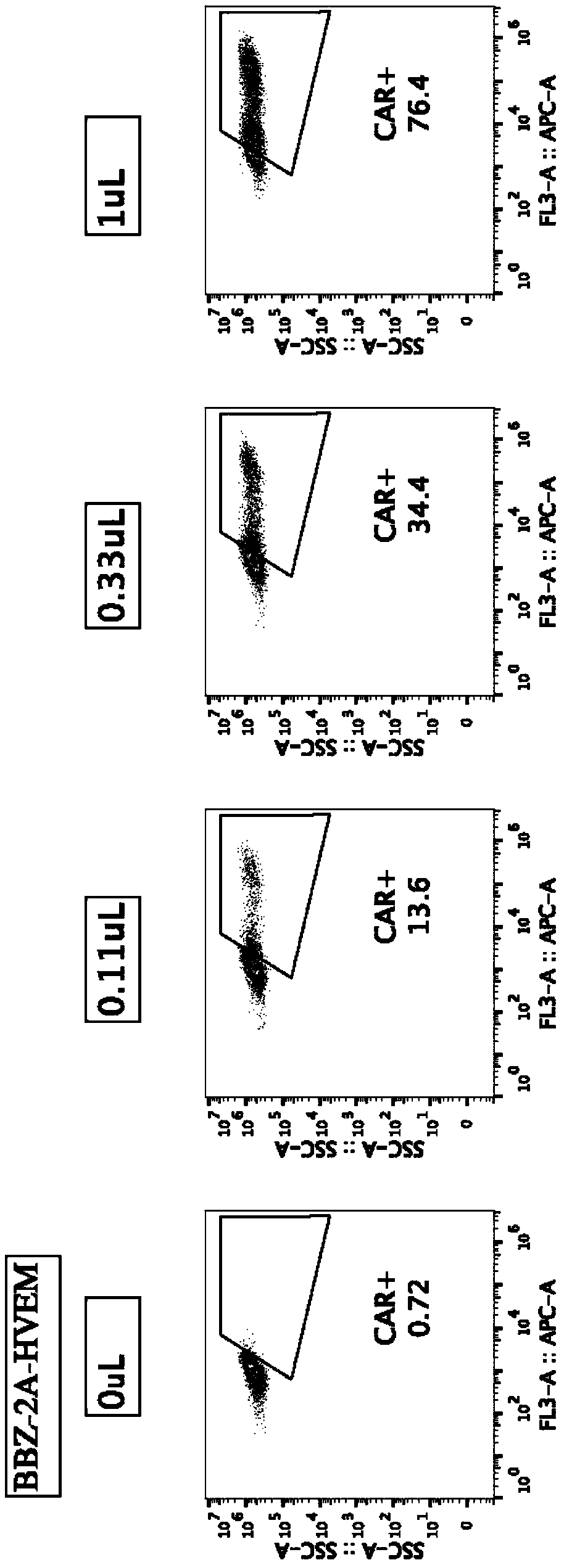
Embodiment 2-20B
[0080] Example 2-20 Preparation of BBZ-2A-HVEM CAR-T cells
[0081] The preparation of 20BBZ-2A-HVEM CAR-T cells described in this example includes the following steps:
[0082] 1. Construction of lentiviral vector pCDH-MSCVEF-20BBZ-2A-HVEM and virus production
[0083] Add 2A (SEQ ID No.8) sequence to the middle of scFv-antihCD20-20BBZ (SEQ ID No.1) and HVEM (SEQ ID No.3) through overlap PCR, and add EcoRI and SalI restriction sites at both ends to clone pCDH - MSCVEF vector. The clones with correct sequencing were extracted without endotoxin, and co-transfected with lentiviral packaging plasmids (VSV-g, pMD Gag / Pol, RSV-REV) at 293X. After 48 and 72 hours, the supernatant was collected, filtered at 0.45uM, and used Beckman ultracentrifuge and SW28 rotor, 25000RPM centrifugation for 2 hours to concentrate the virus, which is pCDH-MSCVEF-20BBZ-2A-HVEM virus (abbreviated as 20BBZ-2A-HVEM virus), for subsequent CAR-T cell production. Produce contrast pCDH-MSCVEF-20BBZ virus (...
Embodiment 3-20B
[0086] Example 3-20 Preparation of BBZ-2A-ICOS CAR-T cells
[0087] The preparation of 20BBZ-2A-ICOS CAR-T cells described in this example includes the following steps:
[0088] 1. Construction of lentiviral vector pCDH-MSCVEF-20BBZ-2A-ICOS and virus production
[0089] Add 2A (SEQ ID No.9) sequence to the middle of scFv-antihCD20-20BBZ (SEQ ID No.1) and ICOS (SEQ ID No.4) through overlap PCR, and add EcoRI and SalI restriction sites at both ends to clone pCDH - MSCVEF vector. The clones with correct sequencing were extracted without endotoxin, and co-transfected with lentiviral packaging plasmids (VSV-g, pMD Gag / Pol, RSV-REV) at 293X. After 48 and 72 hours, the supernatant was collected, filtered at 0.45uM, and used Beckman ultracentrifuge and SW28 rotor, 25000RPM centrifugation for 2 hours to concentrate the virus, which is pCDH-MSCVEF-20BBZ-2A-ICOS virus (abbreviated as 20BBZ-2A-ICOS virus), for subsequent CAR-T cell production. Produce contrast pCDH-MSCVEF-20BBZ virus (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com