Preparation method of cefepime hydrochloride with reduced content of genotoxic impurity 2-mercaptobenzothiazole
A technology of cefepime hydrochloride and cefepime hydrochloride, which is applied in the field of preparation of cefepime hydrochloride, can solve the problems of drug safety threats, strong toxicity, and economic losses of pharmaceutical factories
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
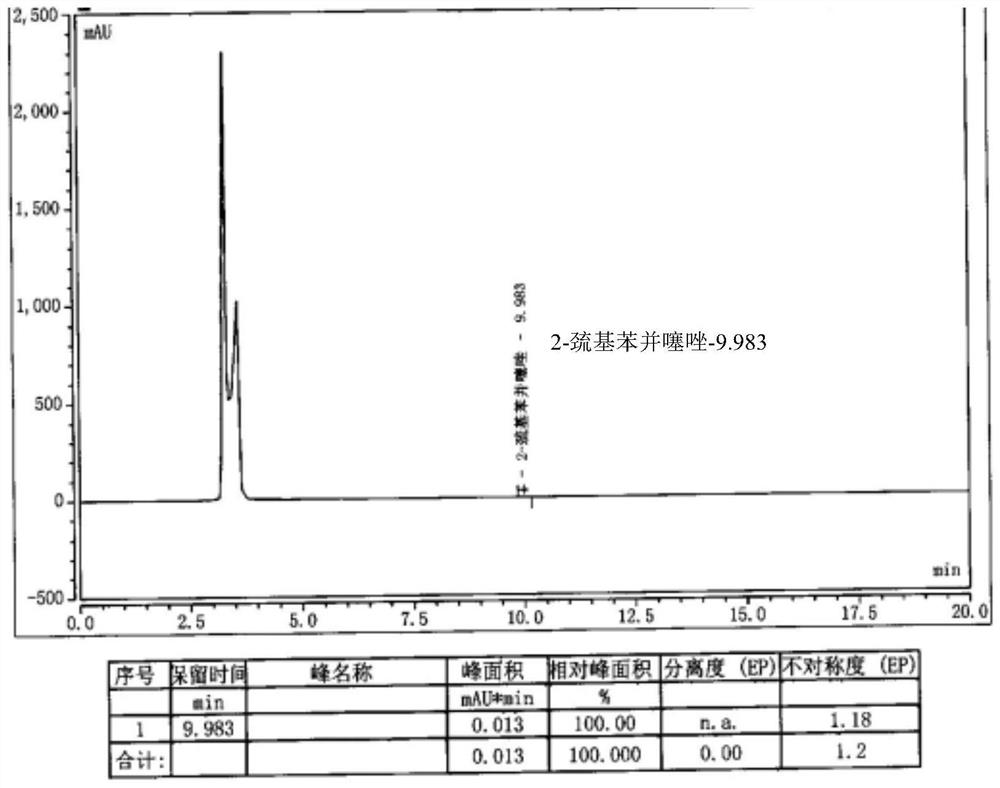
[0032] Add 10g of cefepime side chain compound into 40ml of dichloromethane, add 8.3ml of triethylamine, stir and react for half an hour, add 9.46g of AE active ester to the solution, stir and react for 2 hours at 25-28°C; then, the reaction Pour the liquid into 200ml water, extract, stand and stratify, add 5wt% dilute hydrochloric acid to the obtained water phase, adjust the pH of the water phase to 2.4-2.5, add 20ml×2 toluene to the water phase for extraction twice, and separate the water phase; Add 1g of activated carbon to the water phase, stir and decolorize at room temperature for 20 minutes, filter, wash the activated carbon layer with 10ml of water, and combine the water phases; add 5wt% dilute hydrochloric acid to the combined water phase after decolorization to adjust the pH of the water phase to 1.0-1.1, and then slowly 105ml of acetone was added dropwise, and a large amount of white precipitate was precipitated to obtain 14.4g of cefepime hydrochloride with a molar ...
Embodiment 2
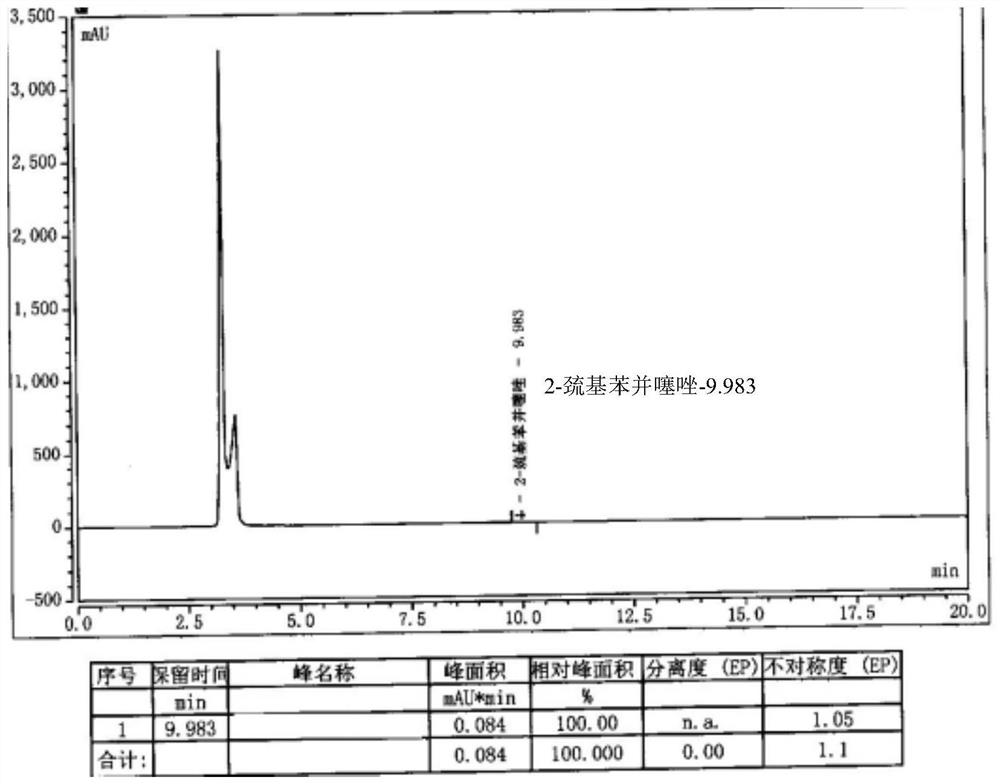
[0045] Add 10g of cefepime side chain compound into 60ml of dichloromethane, add 10ml of triethylamine, stir and react for half an hour, add 10.5g of AE active ester to the solution, stir and react for 2 hours at 25-28°C; then, the reaction solution Pour it into 240ml of water, carry out extraction, let it stand for stratification, add 12wt% dilute hydrochloric acid to the obtained water phase, adjust the pH of the water phase to 2.0-2.1, add 24ml×2 xylene to the water phase for extraction twice, and separate the water phase; Add 2g of activated carbon to the water phase, stir at room temperature for 20 minutes to decolorize, filter, wash the activated carbon layer with 20ml of water, and combine the water phase; add 12wt% dilute hydrochloric acid to the water phase after decolorization to adjust the pH of the water phase=1.4-1.5, and then slowly 260ml isopropanol was added dropwise, and a large amount of white precipitates were separated out to obtain cefepime hydrochloride 14...
reference example 1
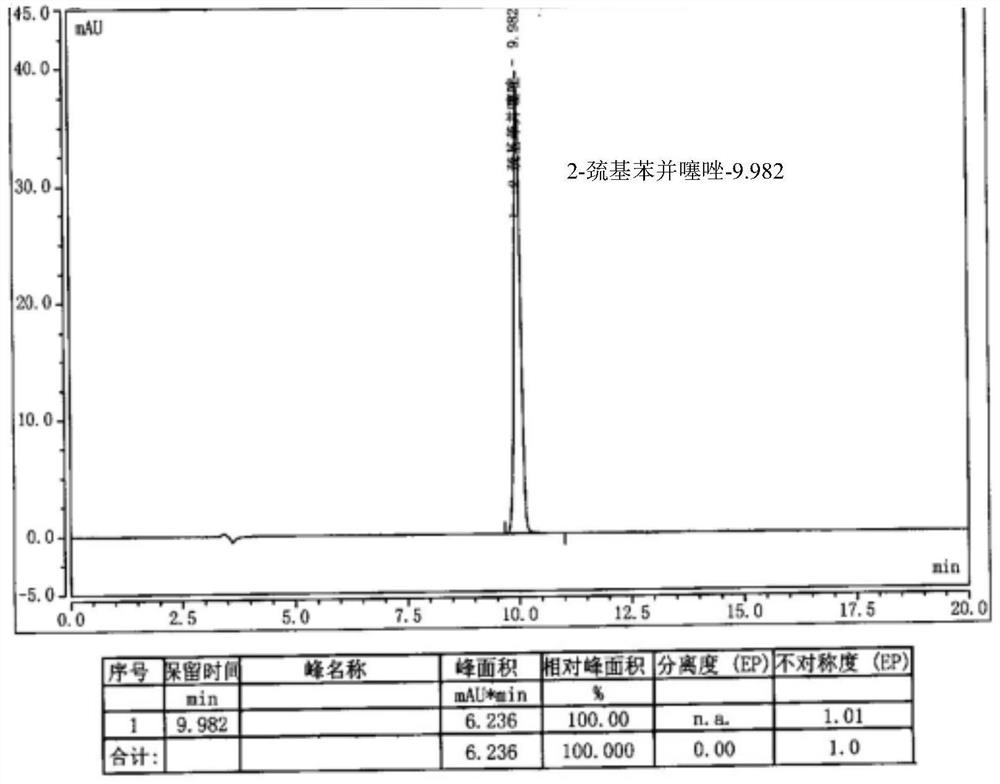
[0047] Dissolve 8g of cefepime side chain compound in 40ml of dichloromethane, add 10ml of triethylamine, stir and react for half an hour, add 7.4g of AE active ester to the solution, stir and react for 2 hours at 28°C; then, pour the reaction solution into 200ml of water, extract, stand for stratification, add 5wt% dilute hydrochloric acid to the obtained water phase, adjust the pH of the water phase to about 1.15, add 20ml of ethyl acetate to the water phase, extract, stand for stratification, and separate the water phase Add 1g of activated carbon to the water phase, stir and decolorize at room temperature for 20 minutes, filter, wash the activated carbon layer with 10ml water, and merge the water phase; slowly add 100ml of acetone dropwise to the combined water phase after decolorization, and a large amount of white precipitates are separated out to obtain cephalosporin hydrochloride Piroxime 11.0g, molar yield 83%, product purity 99.2%, 2-mercaptobenzothiazole content 14mm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com