Immune sensitization predicted biomarker component, use and kit equipment storage medium
A biomarker and composition technology, applied in the biological field, can solve the problems of expensive monoclonal antibody, heavy economic burden, and unsatisfactory precision treatment, and achieve the effect of saving treatment time and money, and effective economic performance reference indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
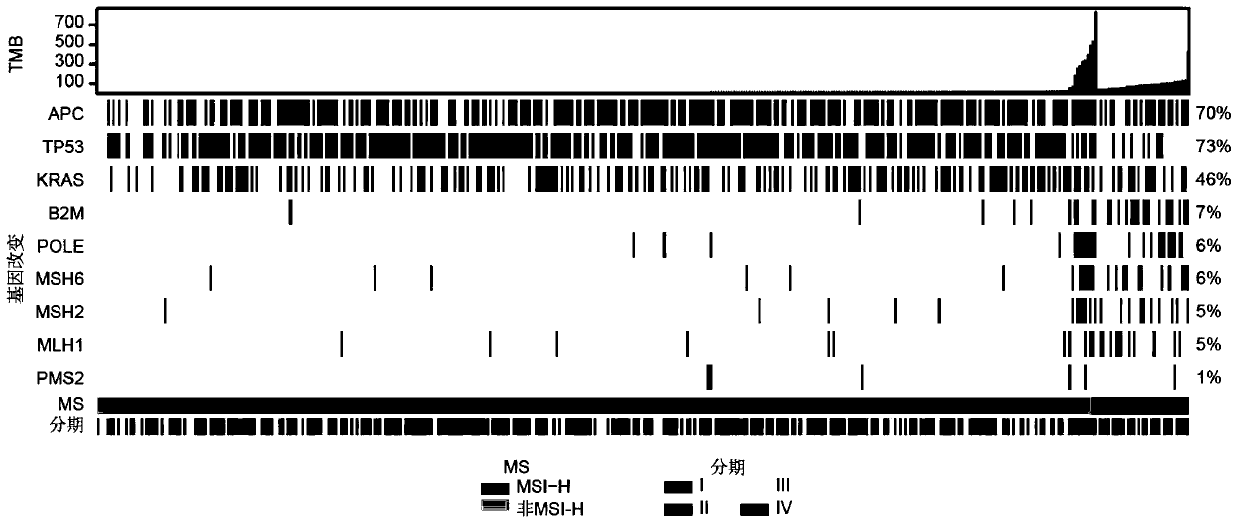
[0097] In this example, through the analysis of specific colorectal cancer subjects (CRC), the biomarker composition of the present invention that can be used to predict the sensitivity of colorectal cancer subjects to immune checkpoint inhibitors is specifically illustrated.
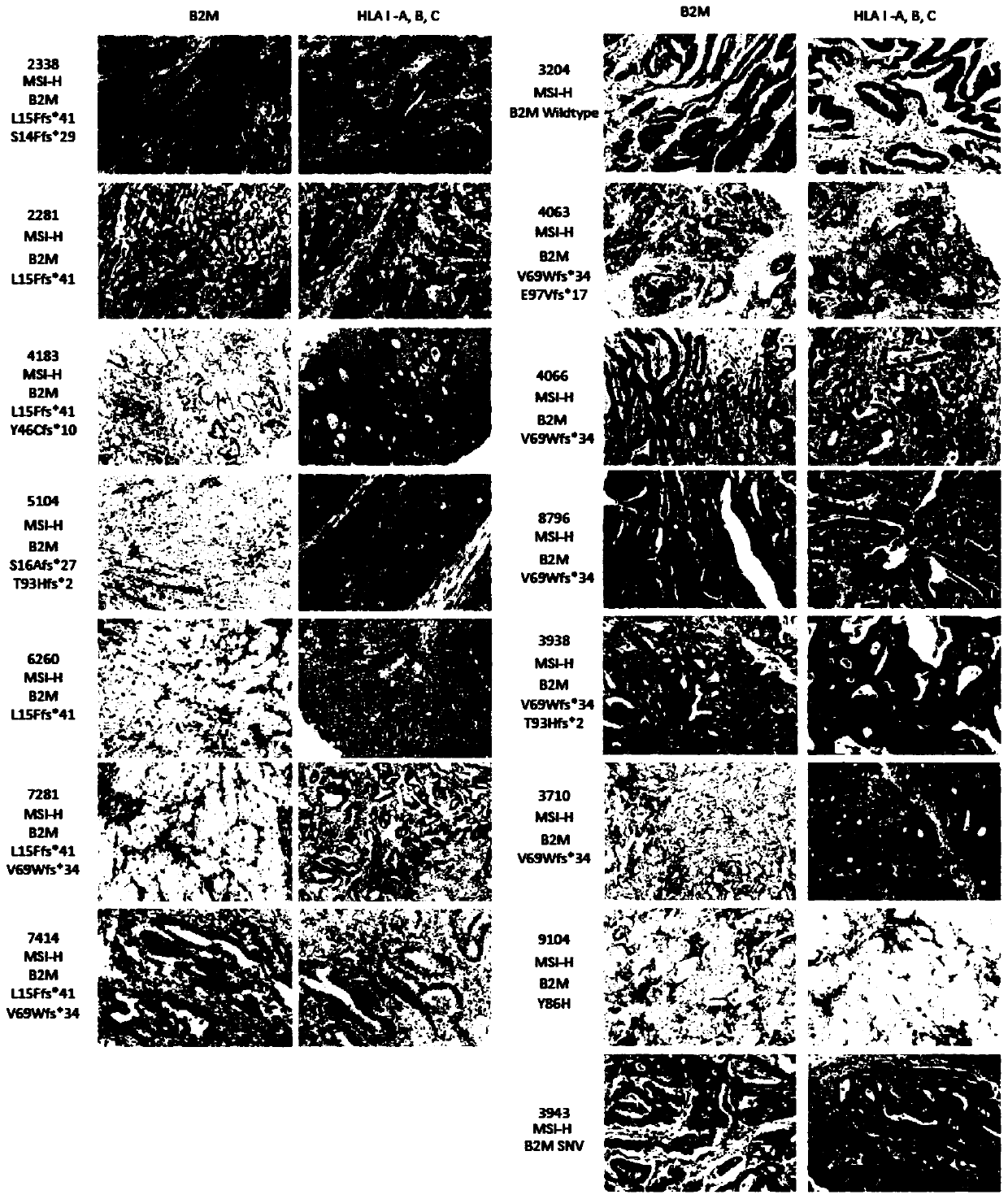
[0098] In this embodiment, a total of 426 CRCs were analyzed, and the object information is shown in Table 1, including 256 males (median age=61) and 170 females (median age=59), whose FFPE tumor samples (tissue samples) and matched whole blood samples (control samples) were subjected to genome-wide analysis as above with a 450-gene panel (panel) and genome-wide analysis, among which, most CRC (50%) were stage IV, 24% were stage III, 20% were stage II and 6% were stage I. The incidence of MSI-H determined by PCR and based on mutation information was 9% (38 / 426), while among MSI-H CRC subjects, only 18% (7 / 38) of MSI-H CRC subjects were at advanced stage, while most Non-MSI-H CRC subjects (53%) exhibite...
Embodiment 2
[0148] This example illustrates a kit for the prediction of sensitivity to immune checkpoint inhibitors.
[0149] In this embodiment, the extraction and quantification of DNA in the sample can be completed by using corresponding kits.
[0150] In addition, in order to make the follow-up prediction more accurate, the quality of the tissue samples is judged first before the tissue samples are constructed and sequenced, and the qualified tissue samples are then subjected to subsequent steps such as sequencing. For this reason, this embodiment provides A kit for detecting a biomarker composition for predicting a subject's sensitivity to an immune checkpoint inhibitor for colorectal cancer, including primers for amplifying the ACTIN gene to determine whether a tissue sample from the subject is of acceptable quality , and when the DNA extracted from the tissue sample can be specifically amplified to the housekeeping gene ACTIN gene by the corresponding predetermined primers, the tis...
Embodiment 3
[0160] This example illustrates a relevant device for immune checkpoint inhibitor sensitivity prediction.
[0161] Image 6 It is the immune checkpoint inhibitor sensitive system involved in Example 3 of the present invention.
[0162] like Image 6 As shown, this embodiment provides an immune checkpoint inhibitor sensitivity prediction system 100 , including a corresponding information obtaining device 10 and an immune checkpoint inhibitor sensitivity prediction device 20 connected through a communication network 30 .
[0163] The relevant information obtaining device 10 is used to obtain the variation information of the biomarker composition used to predict the sensitivity of the subject of colorectal cancer to immune checkpoint inhibitors. For this purpose, the relevant information obtaining device 10 adopts the existing sequencing method, Complete the above sequencing to obtain the sequencing results, and based on the sequencing results, perform the above genome change a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com