Recombinant tau epitope chimeric multimeric antigen, its preparation method and application
A multimer and epitope technology, applied in the fields of biopharmaceuticals and genetic engineering, can solve the problems of inability to effectively neutralize oligomers, failure to achieve the therapeutic effect of improving cognitive ability, and inability to improve cognitive ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
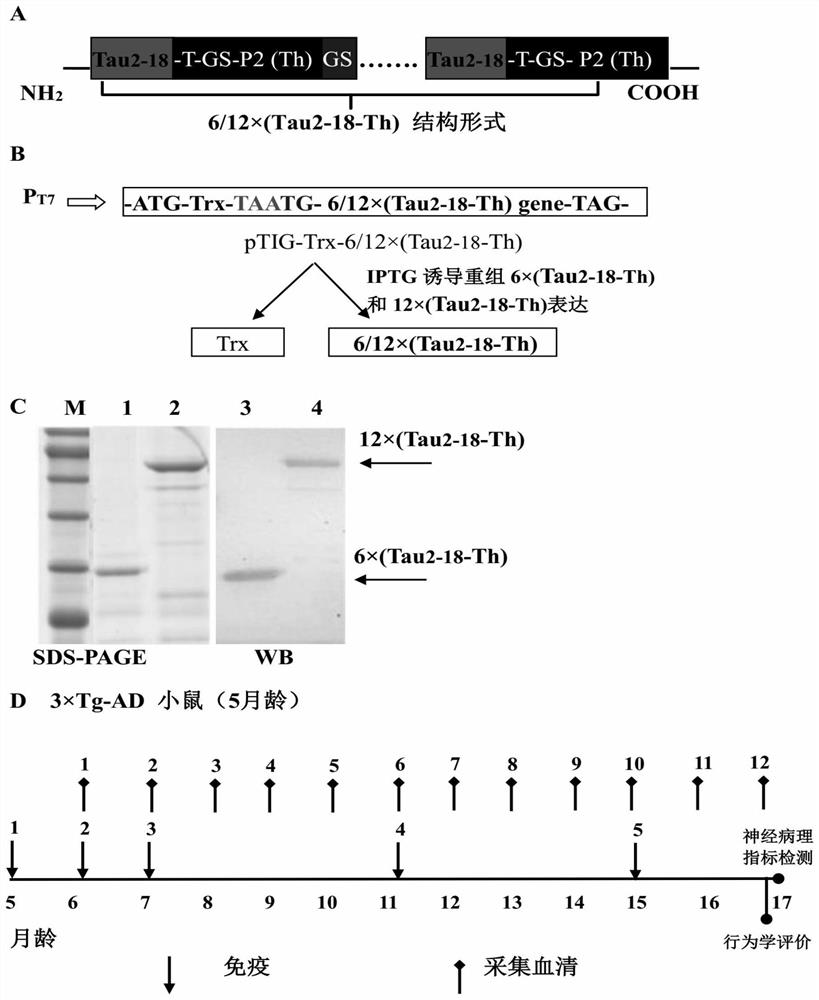
[0069] Embodiment 1, the construction of recombinant prokaryotic expression vector and the expression and purification of recombinant Tau epitope chimeric multimer antigen in Escherichia coli
[0070] 1. Gene design and synthesis of 6×(Tau2-18-Th) and 12×(Tau2-18-Th)
[0071] According to codon degeneracy, the 6×(Tau2-18-Th) gene encoding 6 copies in tandem (see SEQ ID No. 1) and the 12×(Tau2-18-Th) gene in 12 copies in tandem were synthesized artificially (see SEQ ID No. 1). SEQ ID No.2), directly synthesized and cloned into pMD18-T (TaKaRa)T vector named pMD18-6×(Tau2-18-Th) and pMD18-12×(Tau2-18-Th), encoding 6× (Tau2-18-Th) and 12×(Tau2-18-Th) (see SEQ ID No. 3 and SEQ ID No. 4 for the amino acid sequence), each Tau2-18-Th is linked by a GS flexible peptide ( figure 1 middle A). In each Tau2-18-Th molecule, Tau2-18 is the phosphatase-activating domain (PAD) of Tau protein, and the polypeptide Tau2-18 molecule can be used as the B cell epitope of this chimeric multimeric ...
Embodiment 2
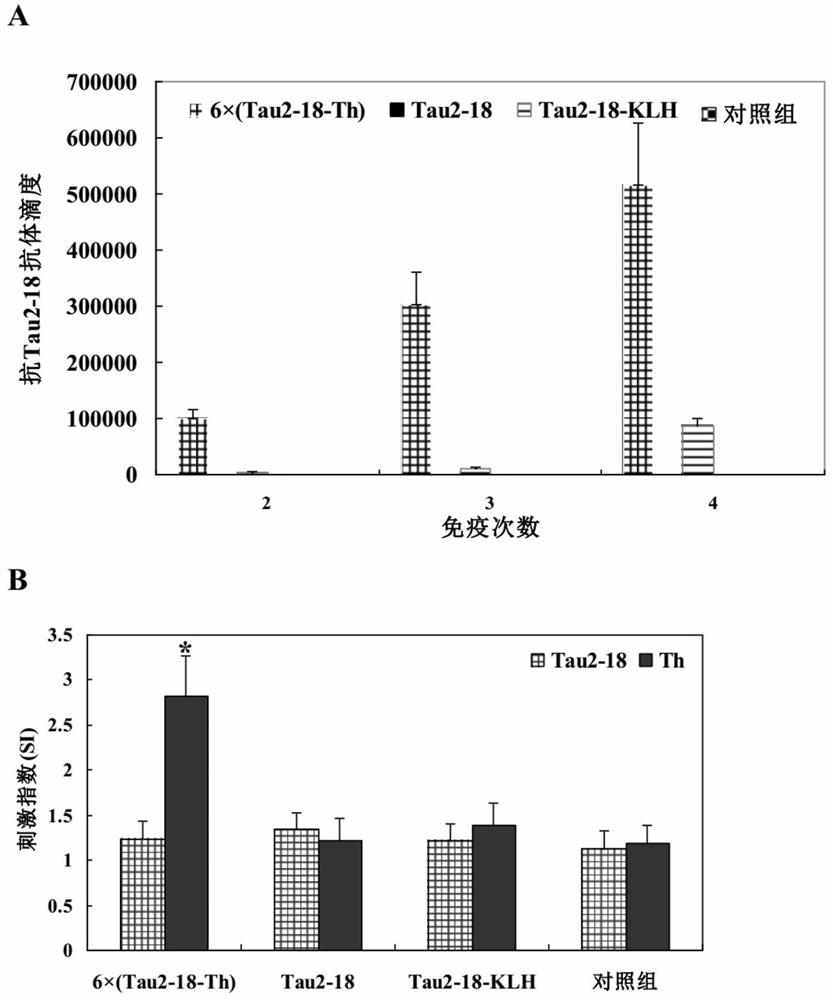
[0082] Example 2. Recombinant Tau epitope chimeric multimer antigen induces high-level Th2-type anti-Tau2-18 antibody response in normal mice
[0083] Immunize mice with recombinant Tau epitope chimeric multimeric antigen proteins 6×(Tau2-18-Th) and 12×(Tau2-18-Th) expressed and purified in Example 1 as immunogens, that is, subunit vaccines to test its immunogenicity. The specific method is as follows: C57 / BL6 mice (8 weeks old, female, SPF grade, Experimental Animal Center of Military Medical Research Institute) were randomly divided into 3 groups, 8 mice in each group, immunized with 10 μg recombinant protein, and the control group (Control) PBS without recombinant protein was immunized four times in total. Before immunization, the antigen was diluted in a final concentration of 10% (v / v) aluminum adjuvant (Alhydrogel) TM , Brenntag Biosector, Frederikssund, Denmark). Each animal was immunized with intramuscular injection of 100 μl. When boosting the immunization, the int...
Embodiment 3
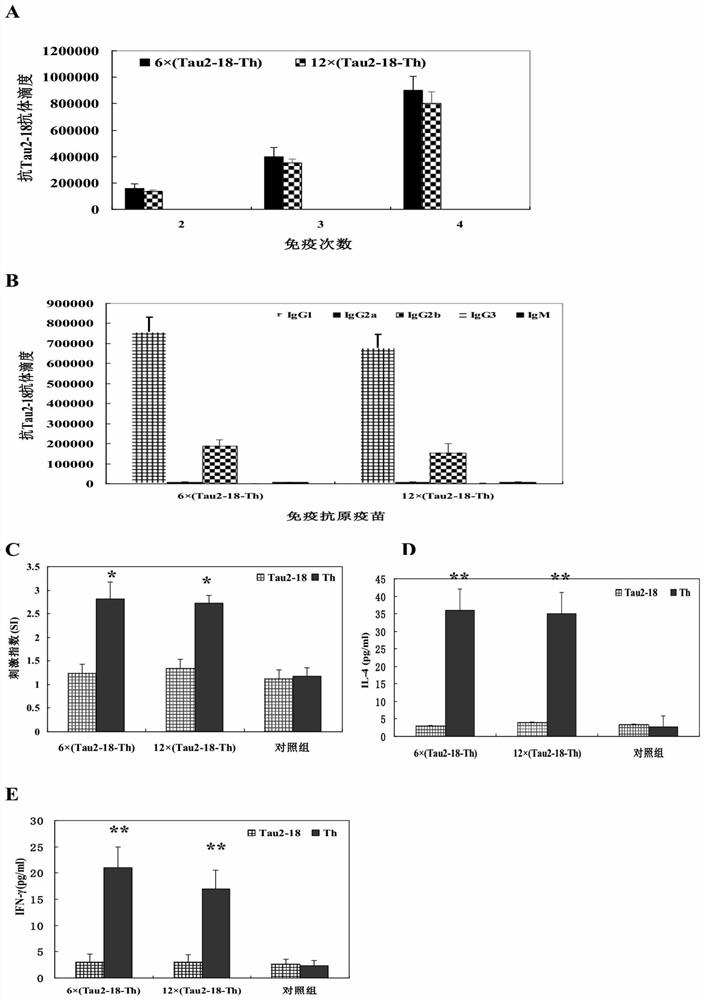
[0087] Example 3. The recombinant Tau epitope chimeric multimer antigen subunit vaccine induces high levels of anti-Tau2-18 antibodies after immunizing 3×Tg AD model mice
[0088] The present invention further evaluates the immunogenicity and immunotherapeutic effect of the recombinant Tau epitope chimeric multimer antigen subunit vaccine by 3×Tg AD model mice (see the scheme). figure 1 middle D). The specific scheme is as follows. The AD model mouse is the 3×Tg AD model mouse, which is knocked in the presenilin protein PS1. M146V Microinjection of genes containing Tau into mice P301L and APP Swe The co-gene sequence of the mouse was constructed to construct an AD pathological model mouse that can express these three proteins. The development of the disease course of this model mouse will show a certain degree of progression with the increase of the age of the mouse, and it will occur at the age of 6 months. Intracellular Aβ deposition, the pathological characteristics of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com