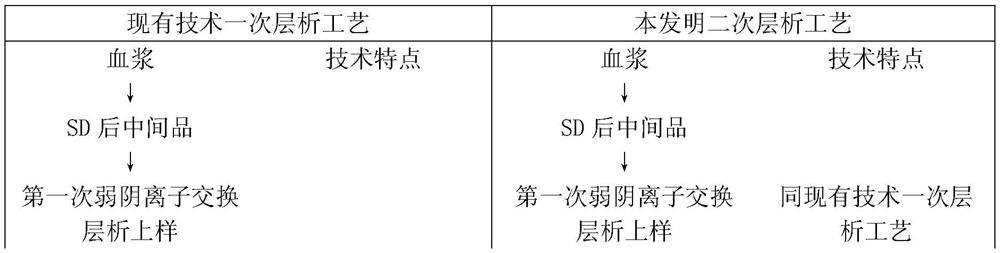
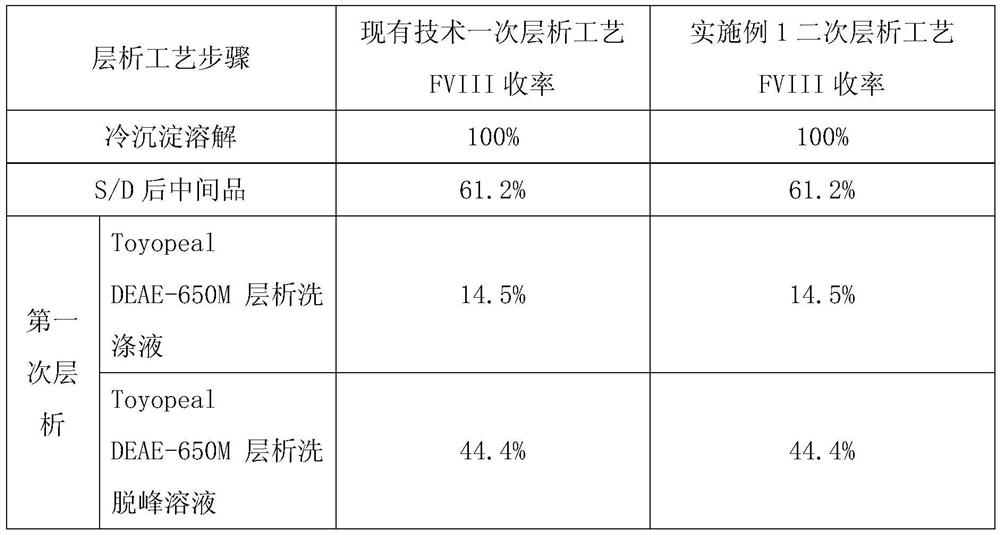
A kind of production process of blood-derived human blood coagulation factor VIII
A technology of human blood coagulation factor and production process, which is applied in the direction of blood coagulation/fibrinolytic factor, VII factor, animal/human protein, etc., can solve the problems of limited yield capacity of FVIII products, and achieve convenient process upgrading, efficient recovery, and cost saving The effect of rate loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] A process for producing blood-derived human coagulation factor VIII,
[0052] (1) Plasma preparation cryoprecipitate, cryoprecipitate dissolution, impurity removal
[0053] Plasma was cryoprecipitated by centrifugation. 100kg of cryoprecipitate was dissolved with 300kg of water for injection at 35°C with stirring. The pH value of the solution was adjusted to 6.6 with 0.1 mol / L acetic acid, and the conductivity was 3.2 mS / cm. A balanced DEAE Sephadex A50 gel was added, and the amount of the A50 gel was 150 g dry gel. After stirring and adsorbing for 45 minutes, the gel and undissolved precipitate were removed by centrifugation; the supernatant after centrifugation was adjusted to pH 6.3 with 0.1 mol / L acetic acid, and the temperature was lowered to 15°C, and the precipitate was separated by centrifugation. FVIII remained in the supernatant.
[0054] (2) S / D virus inactivation
[0055] The supernatant after centrifugation was clarified and filtered through a 0.2 μm f...
Embodiment 2
[0075] A process for producing blood-derived human coagulation factor VIII,
[0076] (1) Plasma preparation cryoprecipitate, cryoprecipitate dissolution, impurity removal
[0077] Plasma was cryoprecipitated by centrifugation. 100kg cryoprecipitate was dissolved by stirring at 25°C with 300kg water for injection. The pH value of the solution was adjusted to 7.0 with 0.1 mol / L acetic acid, and the conductivity was 3.1 mS / cm. A balanced DEAE Sephadex A50 gel was added, and the amount of the A50 gel was 180 g dry gel. After stirring and adsorbing for 45 minutes, the gel and undissolved precipitate were removed by centrifugation; the supernatant after centrifugation was adjusted to pH 6.2 with 0.1 mol / L acetic acid, and the temperature was lowered to 12 °C, and the precipitate was separated by centrifugation. FVIII remained in the supernatant.
[0078] (2) S / D virus inactivation
[0079] The supernatant after centrifugation was clarified and filtered through a 0.2 μm filter e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com