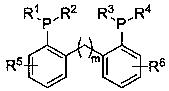
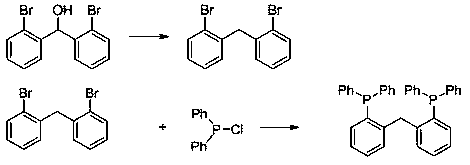
Method for preparing organic carboxylic ester through combined catalysis of aryl bidentate phosphine ligand
A technology of bidentate phosphine ligands and organic carboxylic acid esters is applied in the directions of carbon compound catalysts, chemical instruments and methods, preparation of organic compounds, etc. The synthesis method is simple, good catalytic activity and selectivity, and the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1, methyl propionate
[0025] (1) Preparation of aryl bidentate phosphine ligand a (bis(2-(diphenylphosphino)phenyl)methane)
[0026] Preparation of bis(2-bromophenyl)methanol: under argon atmosphere-15 o At C, a solution of 2-bromoiodobenzene (14.0 g, 50 mmol) in tetrahydrofuran (250 mL) was slowly added dropwise to a solution of isopropylmagnesium chloride lithium chloride (2M THF, 27 mL, 54 mmol). After the exchange was completed, the reaction liquid was cooled to -78°C, and 2-bromobenzaldehyde was added. After the dropwise addition was complete, the reaction temperature was raised to room temperature and stirred for 24 hours. After the reaction, add hydrochloric acid (6M) to quench the reaction, extract with ethyl acetate (3 x 80 mL ), dry the organic phase with anhydrous sodium sulfate, and distill off the solvent under reduced pressure. The resulting mixture is separated by silica gel column to obtain bis(2 -Bromophenyl)methanol (1...
Embodiment 2
[0032] The preparation of embodiment 2, methyl propionate
[0033] (1) Preparation of aryl bidentate phosphine ligand c: The preparation method is the same as that of aryl bidentate phosphine ligand a, except that 2-bromobenzaldehyde is replaced by 2-bromo-5-fluorobenzaldehyde. The structural formula of the aryl bidentate phosphine ligand c is as follows:
[0034]
[0035] (2) Preparation of methyl propionate: use aryl bidentate phosphine ligand c, and the others are the same as in Example 1. The conversion rate of ethylene is 83%, and the selectivity of methyl propionate is 98%.
Embodiment 3
[0036] The preparation of embodiment 3, methyl propionate
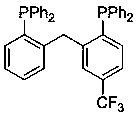
[0037] (1) Preparation of aryl bidentate phosphine ligand e: The preparation method is the same as that of aryl bidentate phosphine ligand a, except that 2-bromobenzaldehyde is replaced by 2-bromo-5-trifluoromethylbenzaldehyde. The structural formula of the aryl bidentate phosphine ligand e is as follows:
[0038]
[0039] (2) Preparation of methyl propionate: aryl bidentate phosphine ligand e was used, and the others were the same as in Example 1. The conversion rate of ethylene is 79%, and the selectivity of methyl propionate is 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com