Preparation method of immobilized lipase
A technology for immobilizing lipase and lipase, which is applied in the direction of immobilization on or in an inorganic carrier, can solve the problems of loss of catalytic activity, mildness, and inability to meet the needs of industrial production, and achieves good stability and cycle times. Multiple, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In the preparation method of immobilized lipase provided by the invention, the amino functionalized magnetic Fe 3 o 4 C nanoparticles were synthesized by the following methods:
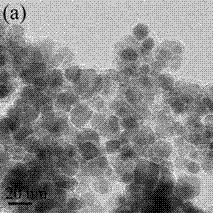
[0033] Weighed 2 mmol of iron acetylacetonate and added it to 60 mL of polyethylene glycol 200, reacted at 270 °C for 3 h in a nitrogen atmosphere, cooled naturally to room temperature, added 4 mmol of glucose, and ultrasonicated for 5 min to obtain a mixed solution; The mixed solution was transferred to an autoclave, and 1 mL of ammonia (NH 3mass percent content, 28%), tighten the hot-pressed reactor, and react at 240 °C for 24 h; after the reaction is completed, it is naturally cooled to room temperature, and the obtained precipitate is magnetically separated, washed, and dried to obtain amino-functionalized magnetic Fe 3 o 4 C nanoparticles, figure 1 For the prepared amino-functionalized magnetic Fe 3 o 4 High-resolution transmission electron microscopy of C nanoparticles.
Embodiment 1
[0035] Step 1. Prepare a 1 mg / mL lipase solution with lyophilized lipase powder derived from Candida rugosa with pH=7.0 phosphate buffer solution, and store it in a refrigerator at 4 °C for later use;
[0036] Step 2. Weigh out 100 mg of amino-functionalized magnetic Fe 3 o 4 C nanoparticles were added to 5 ml of 100g / L glutaraldehyde solution, reacted at 45 °C for 3 h, and the obtained product was magnetically separated, washed, and vacuum-dried;
[0037] Step 3. Take 10 mL of the lipase solution prepared in step 1, add the product obtained in step 2 to it, react at 25 °C for 3 h, and magnetically separate and wash the obtained product to obtain the immobilized fat enzyme.
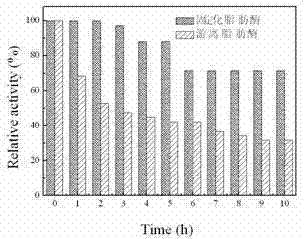
[0038] Detect lipase in the product in magnetic Fe 3 o 4 The immobilized amount on C nanoparticles is 192.7 mg / g; the enzyme activity of the immobilized lipase is 1895.6 U / g; the recovery rate of the enzyme activity of the immobilized lipase is 164%; After standing for 10 h, if figure 2 Shown, the ...
Embodiment 2
[0040] Step 1. Prepare a 1 mg / mL lipase solution from the freeze-dried lipase powder derived from porcine pancreas with pH=7.0 phosphate buffer solution, and store it in a refrigerator at 4 °C for later use;
[0041] Step 2. Weigh out 100 mg of amino-functionalized magnetic Fe 3 o 4 Add C nanoparticles to 10 ml 10g / L glutaraldehyde solution, react at 25 °C for 24 h, and the obtained product is magnetically separated, washed, and vacuum-dried;
[0042] Step 3. Take 50 mL of the lipase solution prepared in step 1, add the product obtained in step 2 to it, react at 35 °C for 0.5 h, and magnetically separate and wash the obtained product to obtain the immobilized fat enzyme.
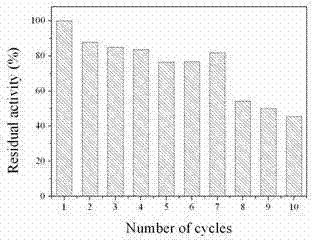
[0043] Detect pancrelipase in the product on magnetic Fe 3 o 4 The immobilized amount on the C nanoparticles is 68.3 mg / g; the enzyme activity of the immobilized lipase is 3839 U / g; the recovery rate of the enzyme activity of the immobilized lipase is 172%; the immobilized lipase After 8 cycles of lipas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com