Selective inhibitors of clinically important mutants of the EGFR tyrosine kinase
A solvate and CHF2 technology, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve the problems of reducing the sensitivity and inefficiency of EGFR inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0481]
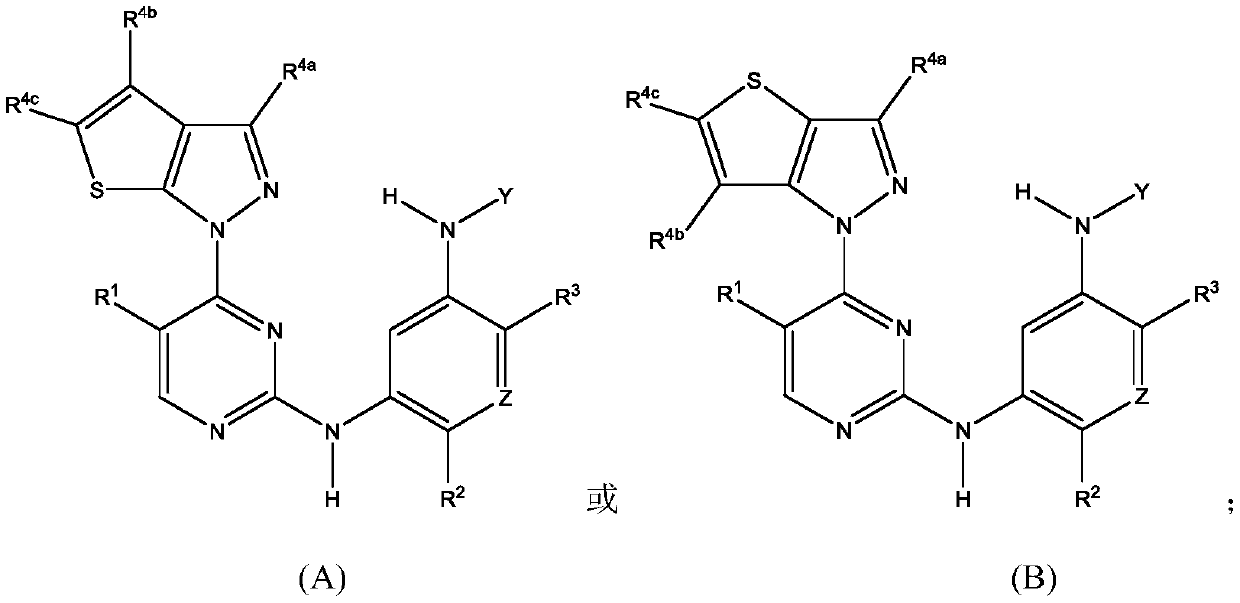
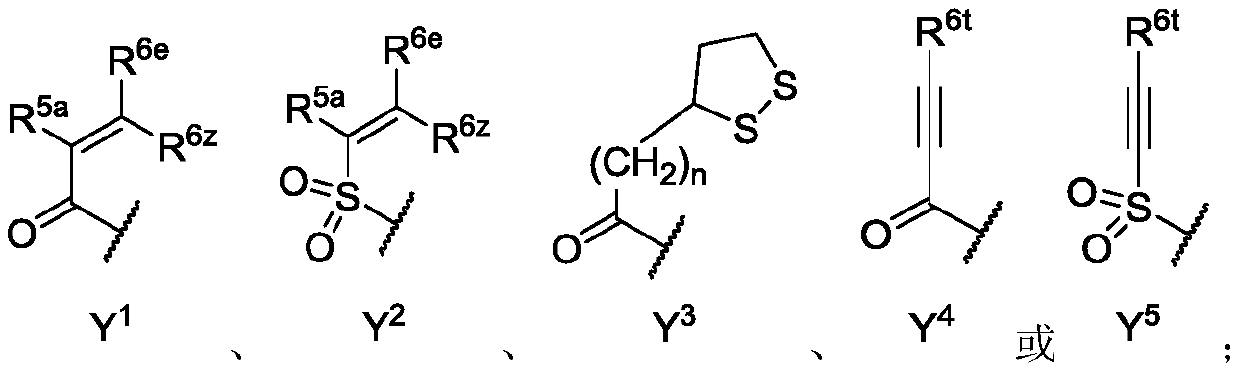
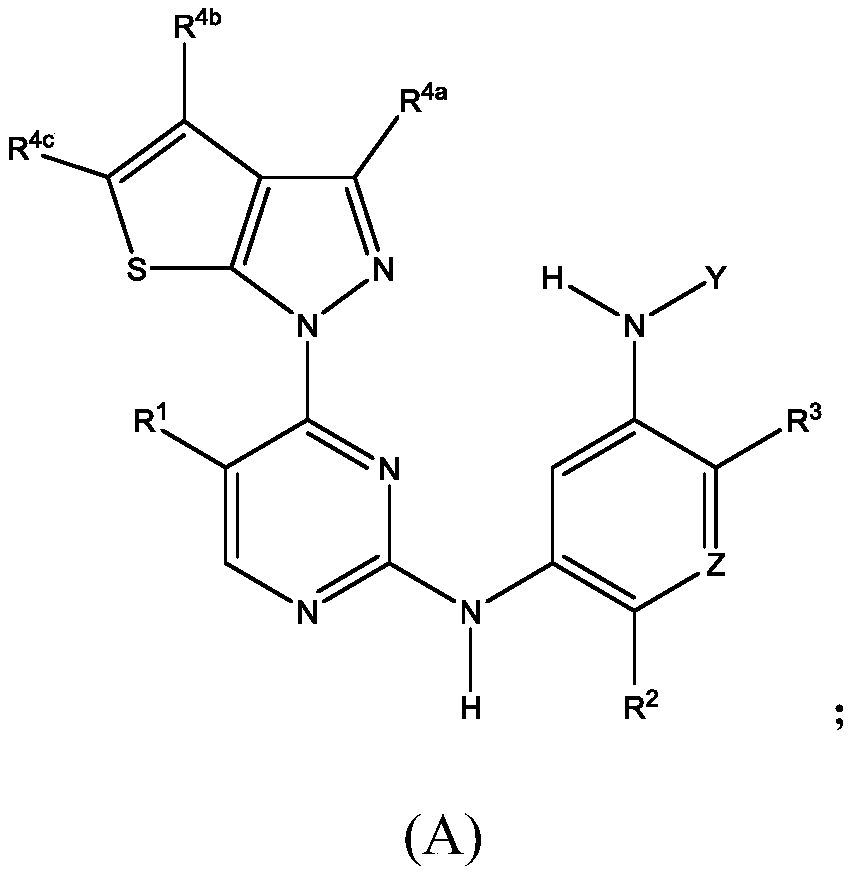
[0482] A1-A4 junction site
[0483] Heteroarylamino linkage microdots
[0484] The following description can be used as A 1 Representative examples of 5,6-bicyclic azaaromatics, but the invention is not limited to these examples:
[0485]
[0486] linking site
[0487]
[0488]
[0489]
[0490] The following description can be used as A 1 、A 2 、A 3 or A 6 Representative examples of 5.X bicyclic azaaromatics, but the invention is not limited to these examples:
[0491] Junction
[0492] The following description can be used as A 3 Representative examples of 5.5 bicyclic azaaromatics, but the invention is not limited to these examples:
[0493]
[0494] linking site
[0495]
[0496] The following description can be used as A 4a or A 4b Representative examples of 6.5 bicyclic azaaromatics, but the invention is not limited to these examples:
[0497]
[0498] linking site
[0499] The following explains A...
example 1
[1313] Example 1. N-(5-((4-(3-(dimethylamino)-6-methyl-1H-pyrazolo[4,3-c]pyridin-1-yl)pyrimidine-2- base)amino)-2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide
[1314]
[1315] 2-Chloro-4-(N,N,6-trimethyl-pyrazolo[4,3-c]pyridin-3-amine-1-yl)pyrimidine (120mg, 0.42mmol, 1.0eq), N- (5-Amino-2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide (134 mg, 0.46 mmol, 1.1 eq) and 2-pentanol (2mL) and p-TsOH·H 2 O (87 mg, 0.46 mmol, 1.1 eq) was sealed in a 10 mL Schlenk tube. The mixture was stirred at 120 °C for 2 h. After completion, the mixture was cooled to RT and washed with saturated NaHCO 3 (10 mL) and DCM / MeOH (10 / 1, 20 mL), the organic layer was separated and the aqueous layer was extracted with DCM (5 mL x 2). Combine the organic layers with NaHCO 3 (20 mL x 2) and brine (20 mL), dried, concentrated and purified by preparative HPLC to provide N-(5-((4-(3-(dimethylamino)-6-methyl-1H- Pyrazolo[4,3-c]pyridin-1-yl)pyrimidin-2-yl)ami...
example 2
[1316] Example 2. N-(5-((4-(7-cyano-1,3-dimethyl-1H-indol-5-yl)pyrimidin-2-yl)amino)-2-((2 -(Dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide
[1317]
[1318] To 2-chloro-4-(7-cyano-1,3-dimethyl-1H-indol-5-yl)pyrimidine (164mg, 0.58mmol, 1.0eq) and N-(5-amino-2 -((2-(Dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide (170mg, 0.58mmol, 1.0eq) in 2-pentanol (4mL) To the solution in , p-toluenesulfonic acid monohydrate (123 mg, 0.64 mmol, 1.1 eq) was added. The mixture was heated to 120° C. for 5 h in a 10 mL Schlenk tube. After cooling to RT, the mixture was poured into water (10 mL), extracted with DCM / MeOH=10:1 (10 mL×3), the combined organic layers were washed with brine (10 mL), dried over sodium sulfate, concentrated and passed Silica column purification affords N-(5-((4-(7-cyano-1,3-dimethyl-1H-indol-5-yl)pyrimidin-2-yl)amino)-2- ((2-(Dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide (48 mg, 15%). 1 H NMR (300MHz, DMSO-d 6 ):δ1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com