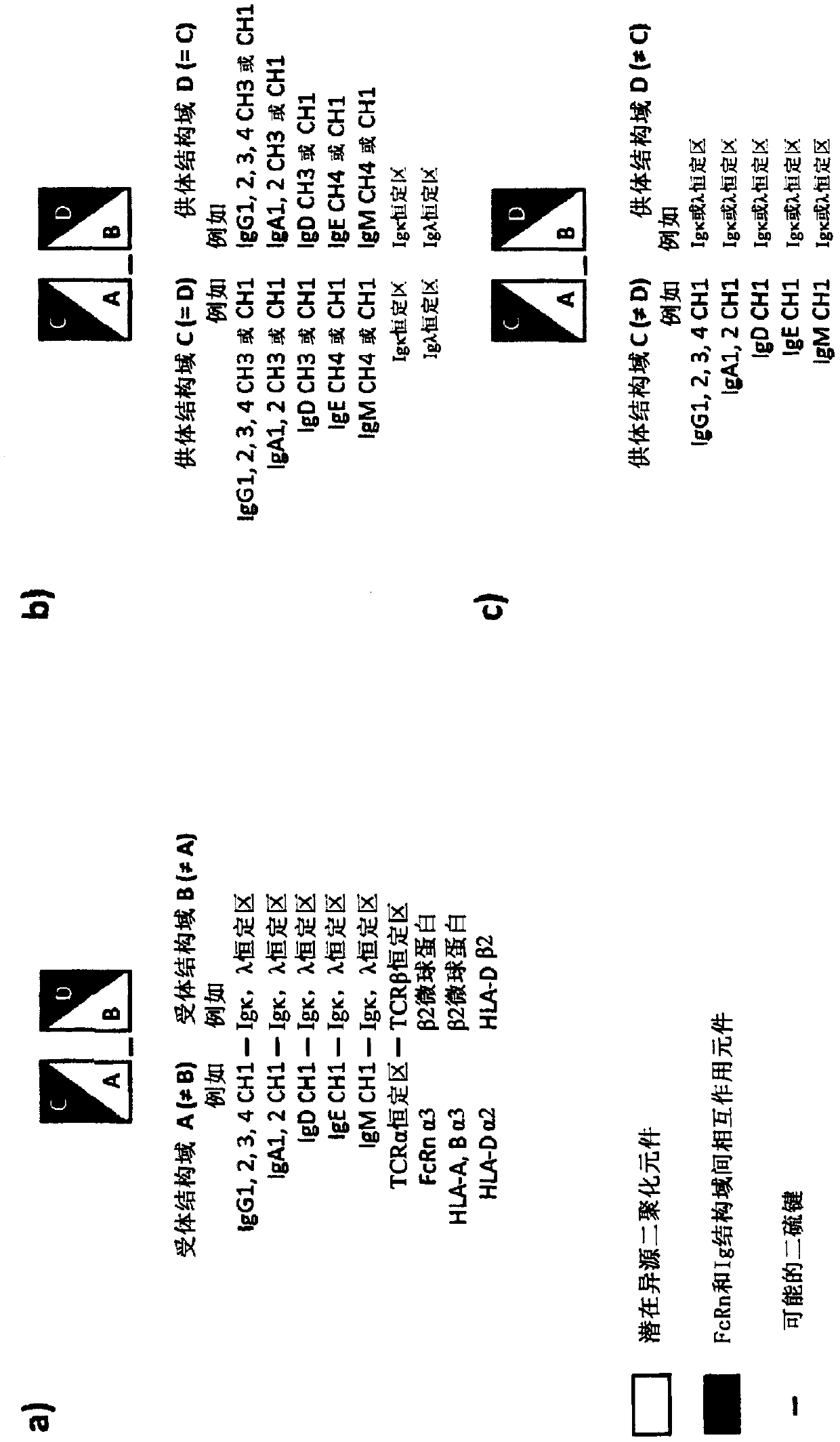
Heterodimerizing Ig domains
A heterodimerization, domain technique, applied to protein complexes, amino acid sequences and/or amino acid sequences of HRII, protein complexes, nucleic acids and vectors, amino acid chains and/or for the prevention, treatment or diagnosis of disorders or diseases The amino acid chain field of HRII can solve problems such as thermal stability and biophysical properties, half-life is not as expected, and the degree of heterodimerization is not satisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0399] Example 1: Heterodimerized Fc Portions
[0400] Dimeric antibody Fc parts were generated using a combination of IgG1 CH1 and Igκ constant domains as the second and fourth CRI, and IgG1 CH3 residues as the first and third CRI sequences, respectively, where for the CH1-CH3 combination (CH31), the sequence composition is 1, 2(#43-51) and 3(#103-132), for the CLk-CH3 combination (CH3k), the sequence composition is 1, 2(#45-51) and 3(# 103-129). In addition to the indicated sequence, residue 37 of CH3 has been transferred to the CLk third CRI sequence, introduced as sequence 1-3, for its potential participation in the structural stabilization of the CH3 element. The sequences of CH31 and CH3k are as Figure 8 shown. To generate a fully functional Fc portion called Fc1k, both domains are fused to the C-terminus of the IgG1 hinge-CH2 domain ( Figure 9 ). To confirm the heterodimerization potential of Fc1k, two molecules were cloned and produced, both comprising scFv13.7 ...
Embodiment 2
[0401] Example 2: Fv13.7-Fc1k
[0402] Separate the variable domains of Fab13.7 through GTG 3 After the SG linker is fused to an Fc chain consisting of CH2 and CH31 or CH2 and CH3k, a Fab-like antibody format comprising a functional bivalent Fc portion is formed ( Figure 12 ). In addition, mutations (A327G, A330S, and P331S, EU numbering) were introduced in CH2 to avoid binding to Fcγ receptors and the complement protein C1q (Richter et al., 2013). After digestion with KpnI and EcoRI, the N-terminal linker (GTG 3 SG), codon-optimized CH2-CH31 and CH2-CH3k (GeneArt TM ) DNA sequence was inserted into pSecTagA-L1 containing VH13.7 or VL13.7.
[0403] Expression of Fv13.7-Fc1k in transiently transfected HEK293-6E cells after co-administration of two plasmids encoding VH13.7-CH2-CH31 or VL13.7-CH2-CH3k using polyethyleneimine as transfection reagent . The protein secreted into the cell culture supernatant was purified by protein A affinity chromatography (14.6 mg / L, see Tab...
Embodiment 3-6
[0409] Examples 3-6: Generation of bivalent or trivalent and bispecific scFv-Fc fusion proteins
[0410] Furthermore, bivalent or trivalent and bispecific scFv-Fc fusion proteins were used to generate bivalent or trivalent and bispecific scFv-Fc fusion proteins to retarget CD3-expressing T cells to FAP-expressing tumor cells or FAP-expressing adult cells using the heterodimerized Fc part Fclk. Tumor cells surrounded by fibroblasts. Thus, scFvhu36 (targeting FAP) and scFvhuU3 (targeting CD3) were partially fused to the N- or C-terminus of Fclk. All constructs were also created using an IgG1 hinge region without cysteine to investigate the importance of hinge-mediated covalent attachment. The resulting construct contained a FAP-targeting portion and a CD3-targeting portion at the N-terminus (FAP N -CH3 N - hFc [with cysteine in the hinge region], Example 3a, and FAP N -CH3 N -F [without cysteine in the hinge region], Example 3b, Figure 17 ), or the resulting constru...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap