Application of triazine tripyrazole compound in anion detection
A technology of triazine tripyrazole and azine tripyrazole, which is applied in the field of anion detection, can solve the problems of unrecognized, unrecognized and the like, and achieves the effect of convenient and quick identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
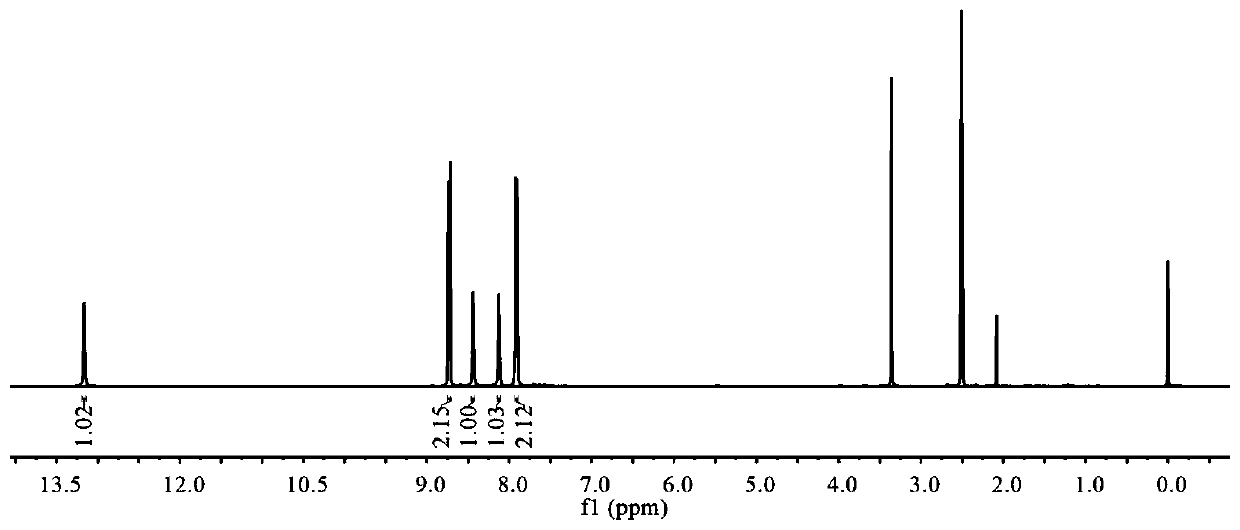
Image
Examples
Embodiment 1
[0024] This embodiment provides a triazine tripyrazole compound with the following structural formula:
[0025]
[0026] Its preparation method comprises the following steps:
[0027] (1) 4-pyrazole boronic acid pinacol ester (10g, 51.5mmol) and 3,4-dihydro-2H-pyran (4.79g, 57mmol) in 25mL toluene solution with trifluoroacetic acid (591mg, 5.2mmol ) as a catalyst, heated and refluxed at 95°C for overnight reaction, and rotary evaporated to obtain 1-(tetrahydropyran-2-yl)-3-(4,4,5,5-tetramethyl-[1,3,2] Dioxin-2-yl)-1H-pyrazole (8.45 g, 19.6 mmol), 59% yield.
[0028] (2) Add 4-bromooxynil (1092mg, 6.0mmol) into a 50mL Slinky bottle, dissolve it with 15mL chloroform solution, slowly add trifluoromethanesulfonic acid (534μL, 2.0mmol) dropwise under ice bath, and Under protection, react at room temperature for 10-15 hours, and then react at 50°C for one day to obtain a white solid (950 mg, 1.74 mmol), namely 2,4,6-tris(4-bromo-phenyl)-1,3,5 - Triazine, yield 87%.
[0029] (...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap