A kind of aggregation-induced luminescent fluorescent material and its preparation method
A technology for aggregation-induced luminescence and fluorescent materials, which is used in the preparation of fluorescent materials, and the field of fluorescent materials with aggregation-induced luminescence properties, which can solve the problems of fluorescence quenching and low solid quantum yield, and achieve the effect of good solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Under nitrogen protection, add 668 μL N,N-dimethylformamide dropwise to 690 μL (8.5 mmol) POCl 3 , stirred at room temperature for 30 min to obtain a red solution, and then added the obtained red solution to 3 mL of N,N-dimethylformamide solution containing 341 mg (1 mmol) N,N-diphenylcoumarin (compound I-1) , stirred the reaction at 60°C for 24h, then added ice water to quench the reaction, adjusted the pH to 5.2 with NaOH, concentrated under reduced pressure, and column chromatography (the eluent was a mixture of petroleum ether and ethyl acetate at a volume ratio of 40:1) liquid), the fluorescent material shown in formula II-1 was obtained, and the yield was 52%.
[0019]
[0020] The structural characterization data of the obtained fluorescent material is: 1 H NMR (300MHz, CDCl 3 )δ10.16(s,1H),8.28(s,1H),7.39(dd,J=12.6,4.9Hz,5H),7.29(s,1H),7.25-7.17(m,5H),6.84(dd ,J=8.8,2.2Hz,1H),6.73(d,J=2.1Hz,1H).
Embodiment 2
[0022] In this example, the N,N-diphenylcoumarin in Example 1 was replaced with an equimolar amount of N,N-xylylcoumarin (compound I-2), and the other steps were the same as in Example 1 , the fluorescent material represented by formula II-2 was obtained, and the yield was 47%.
[0023]
[0024] The structural characterization data of the obtained fluorescent material is: 1 H NMR (300MHz, CDCl 3 )δ10.14(s,1H),8.26(s,1H),7.34(d,J=8.8Hz,1H),7.15(dd,J=28.4,8.3Hz,8H),6.78(dd,J=8.8 ,2.1Hz,1H),6.67(d,J=2.1Hz,1H),2.37(s,6H).
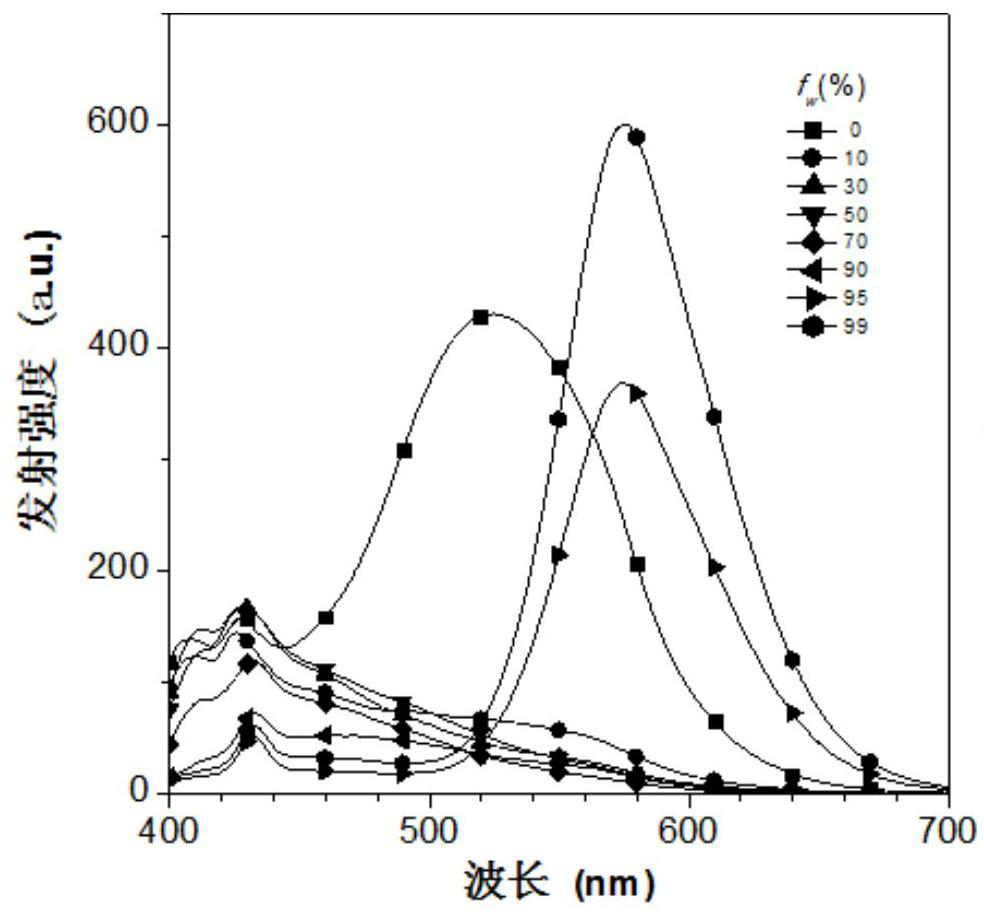
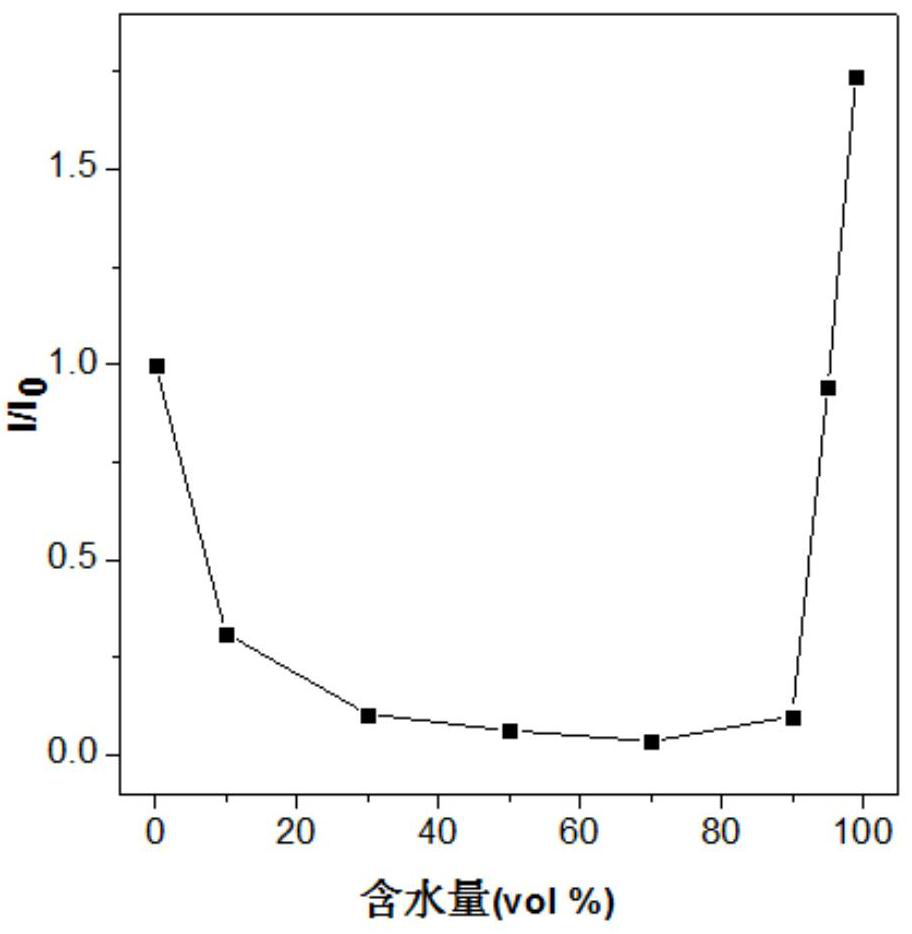
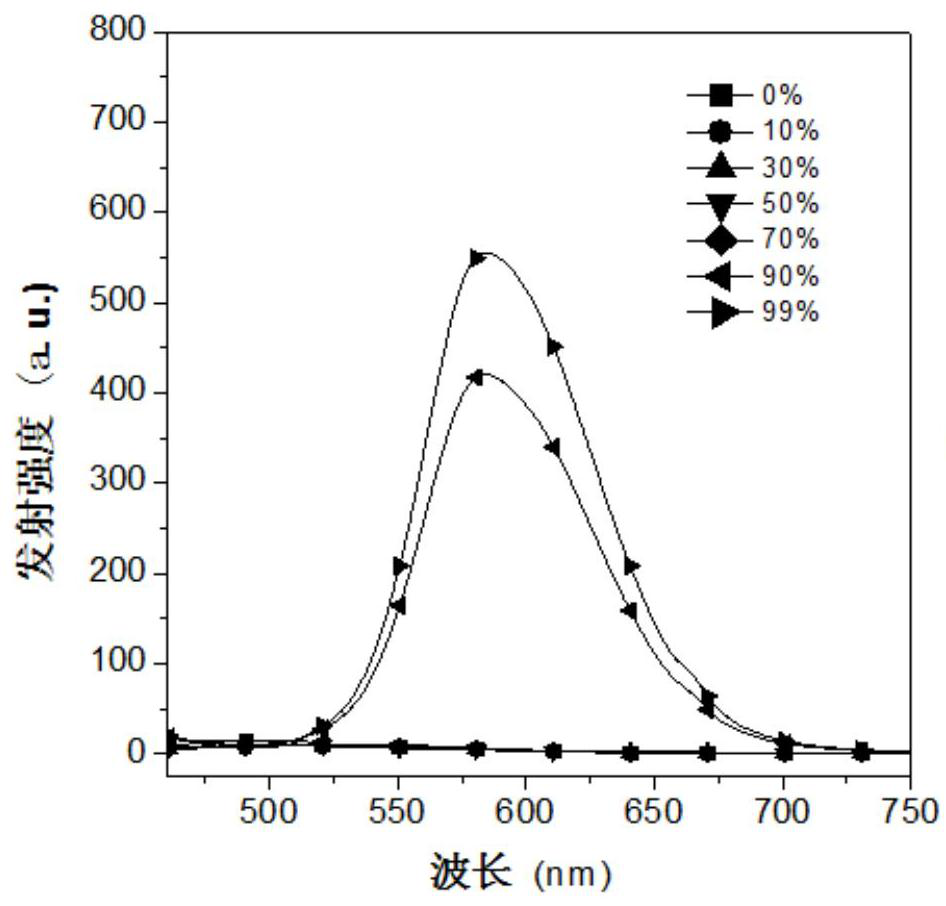
[0025] The inventors tested the aggregation-induced luminescent properties of the fluorescent materials prepared in Examples 1-2 above. %, 99% acetonitrile aqueous solution, adding the fluorescent material into the resulting solution to obtain a concentration of 1×10 -5 mol / L fluorescent material solution, using Hitachi F-7000 fluorescence spectrophotometer to test the aggregation-induced luminescence properties of the fluorescent material, the results a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com