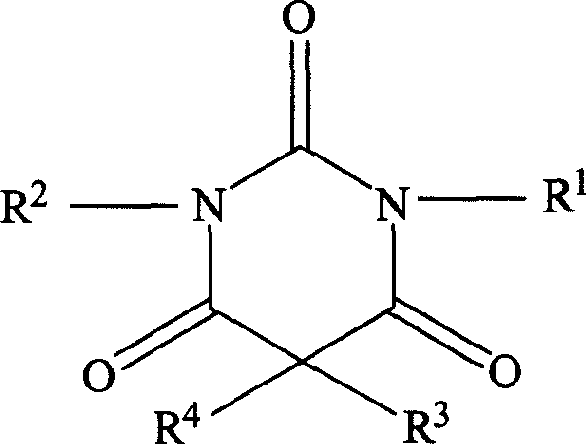
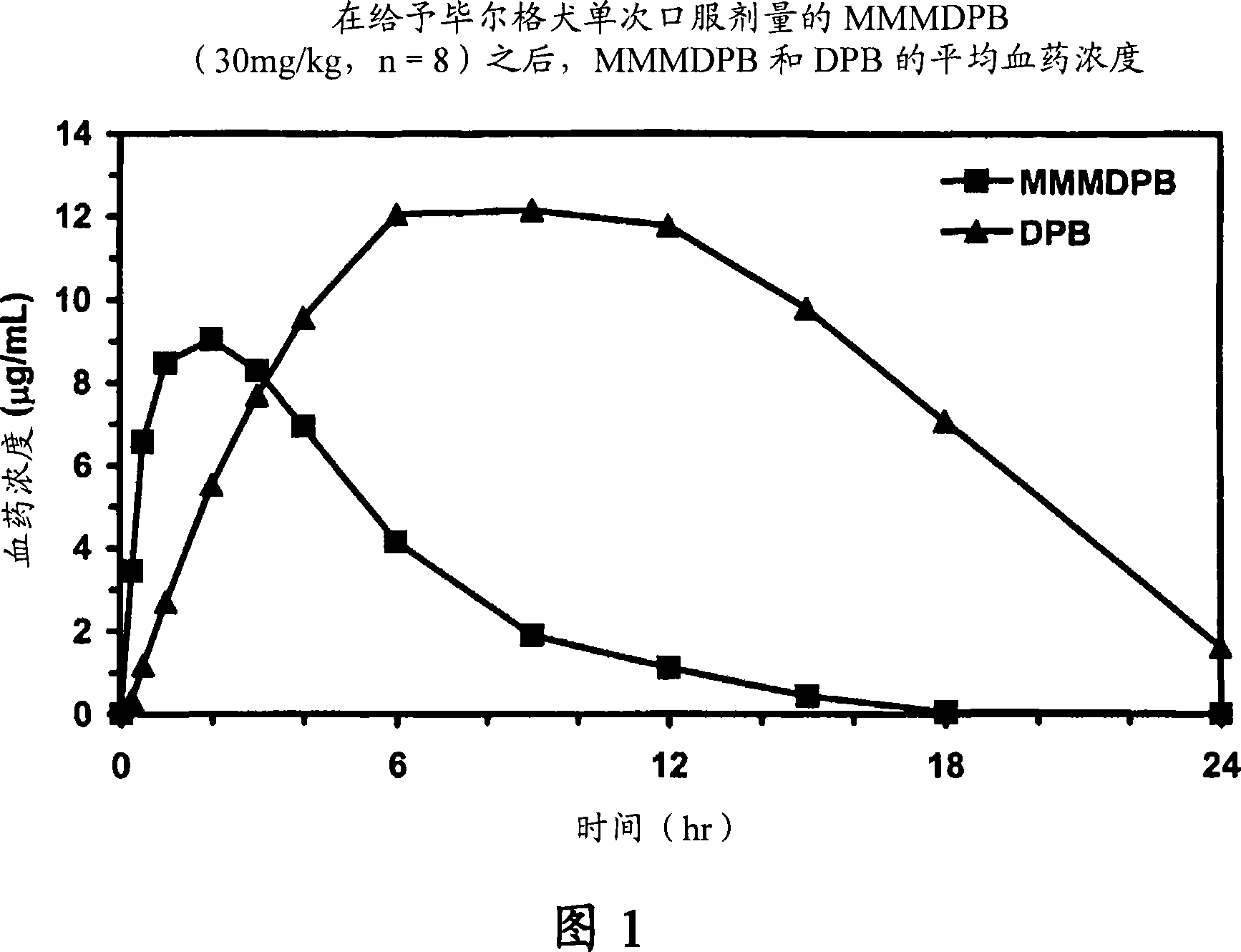
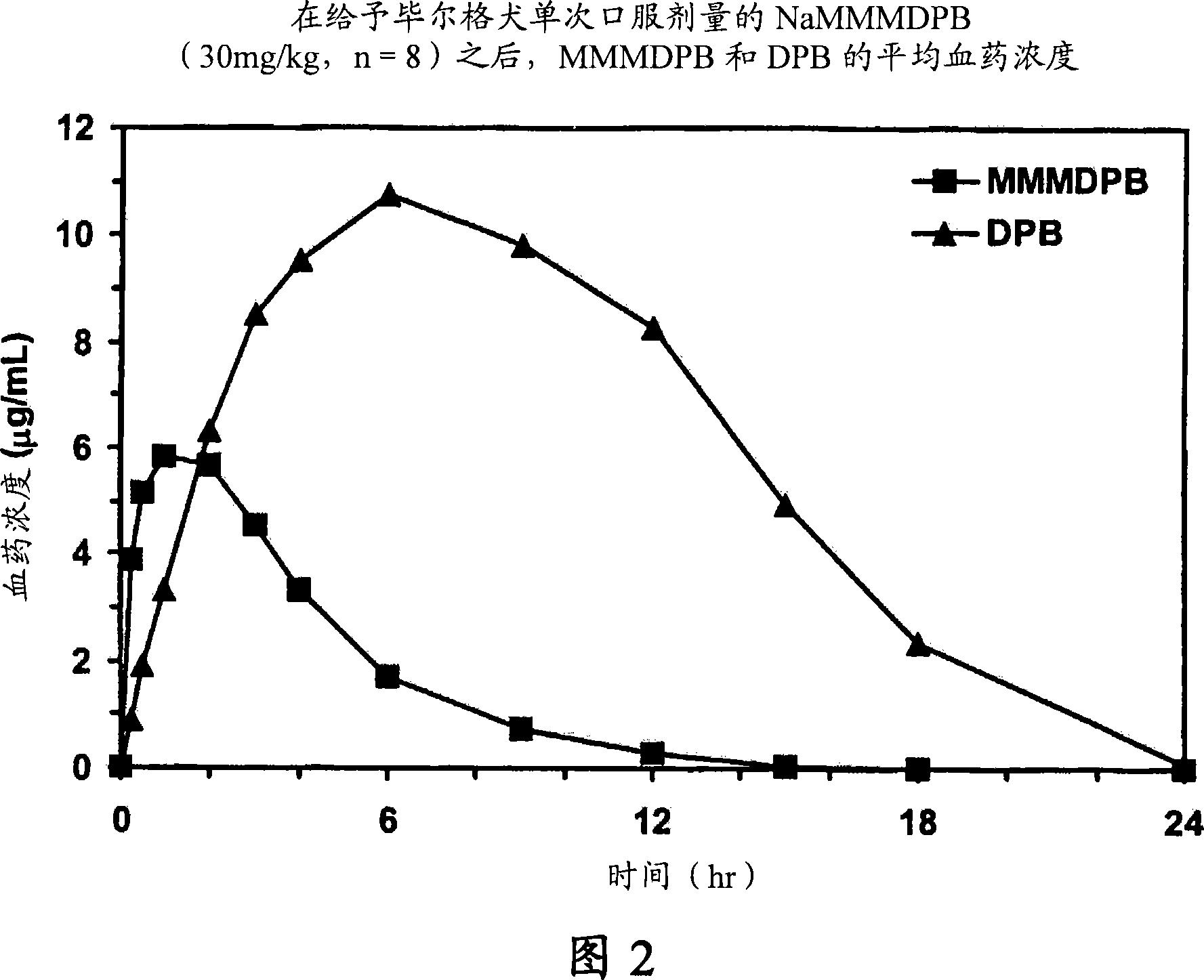
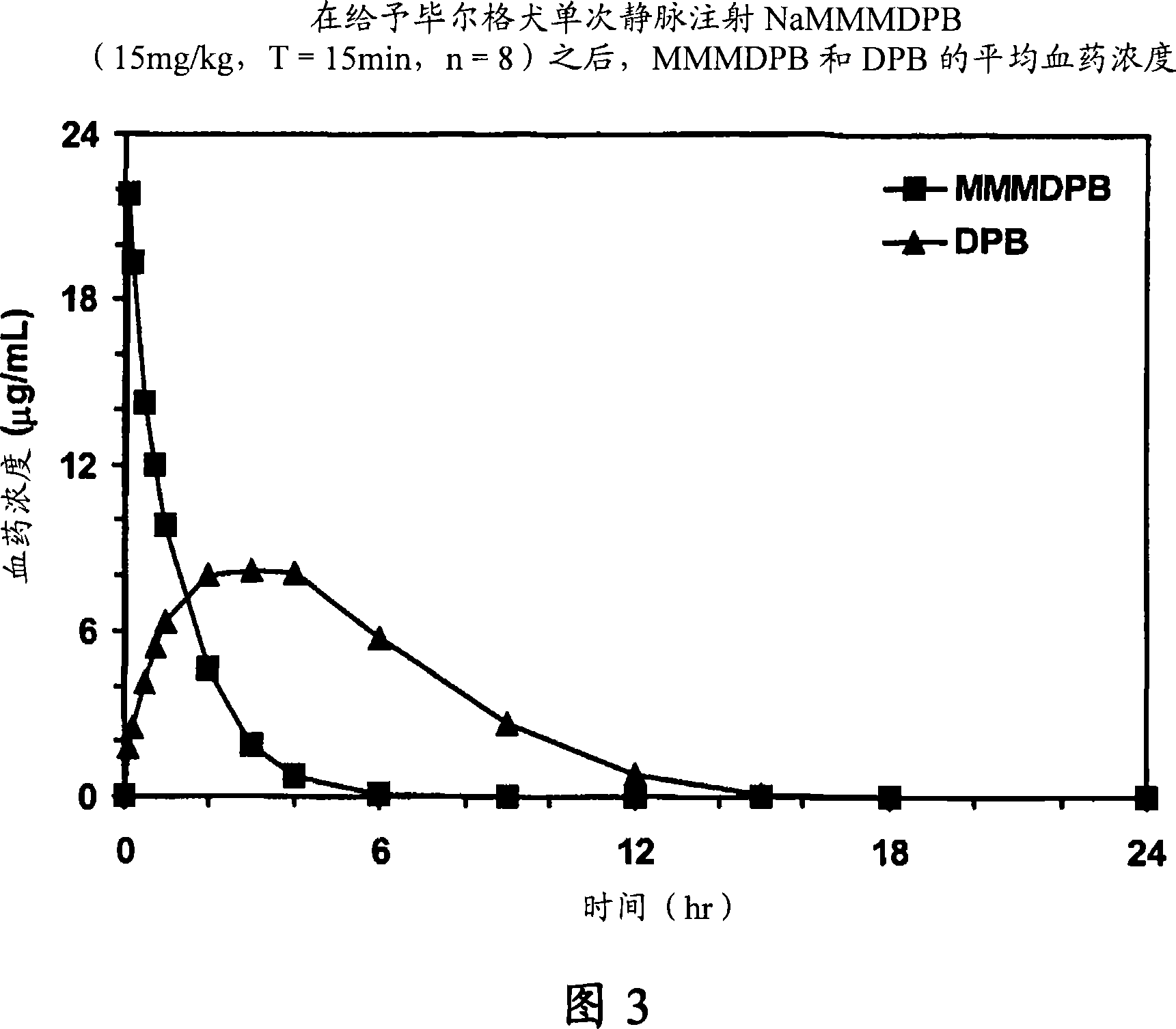
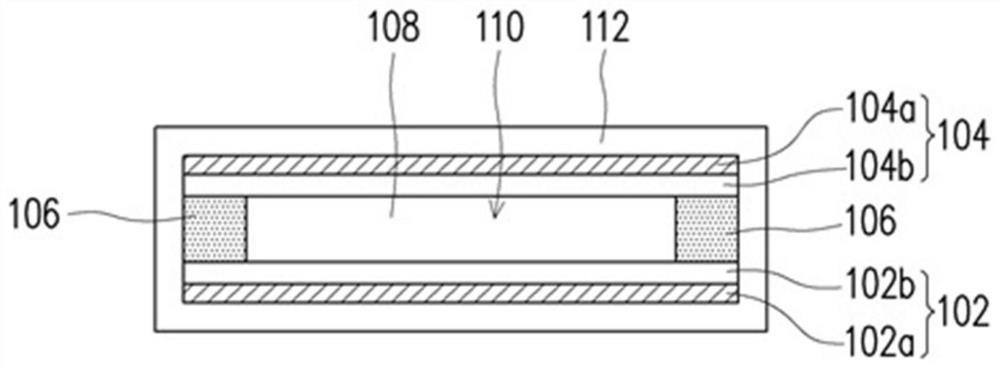
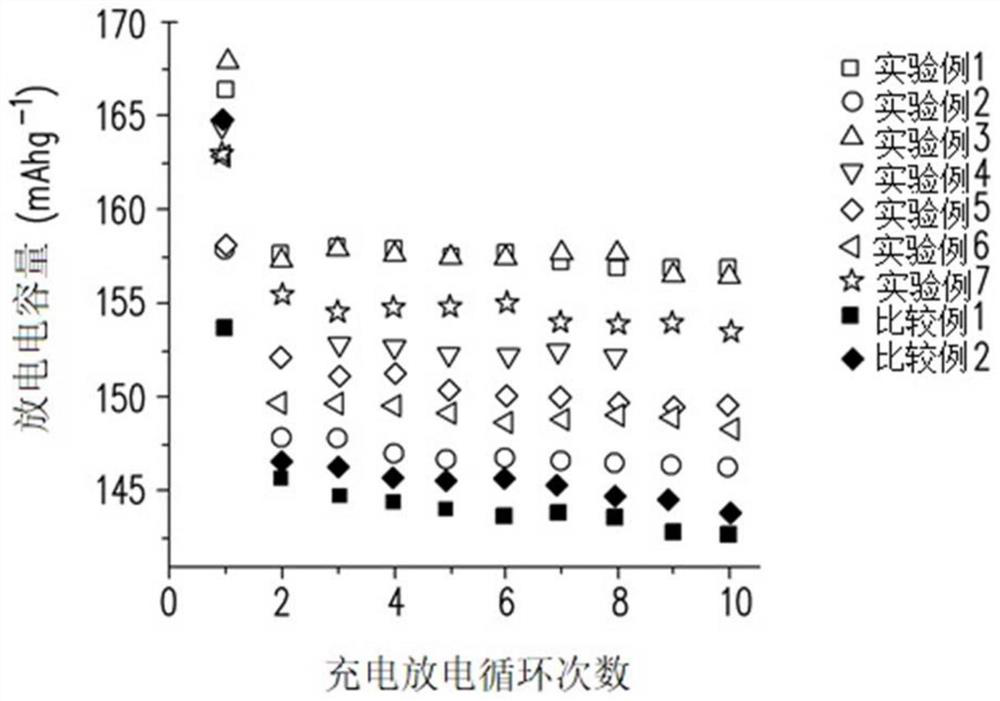
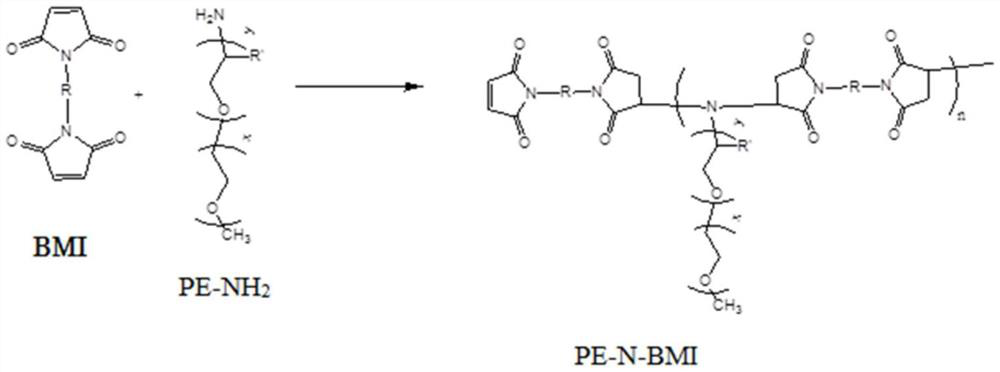
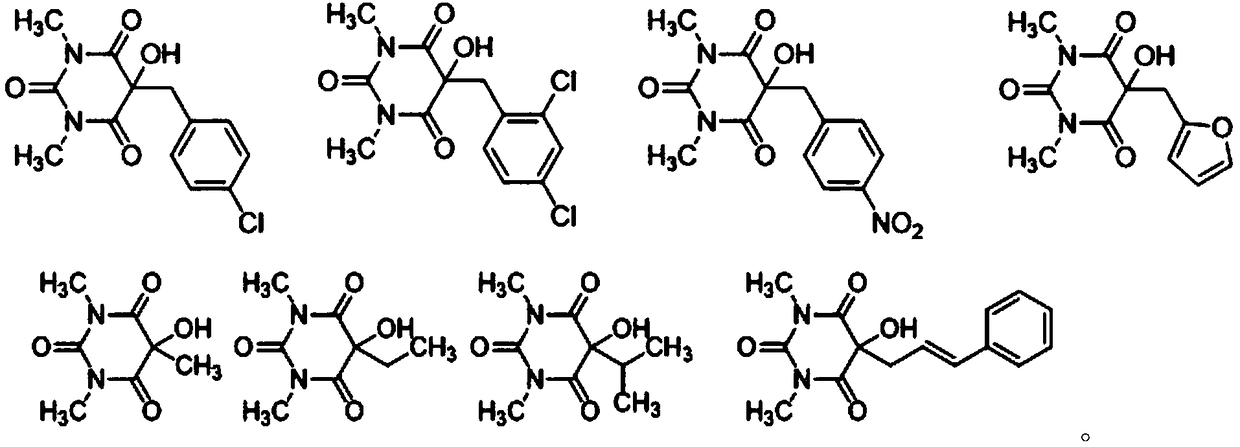
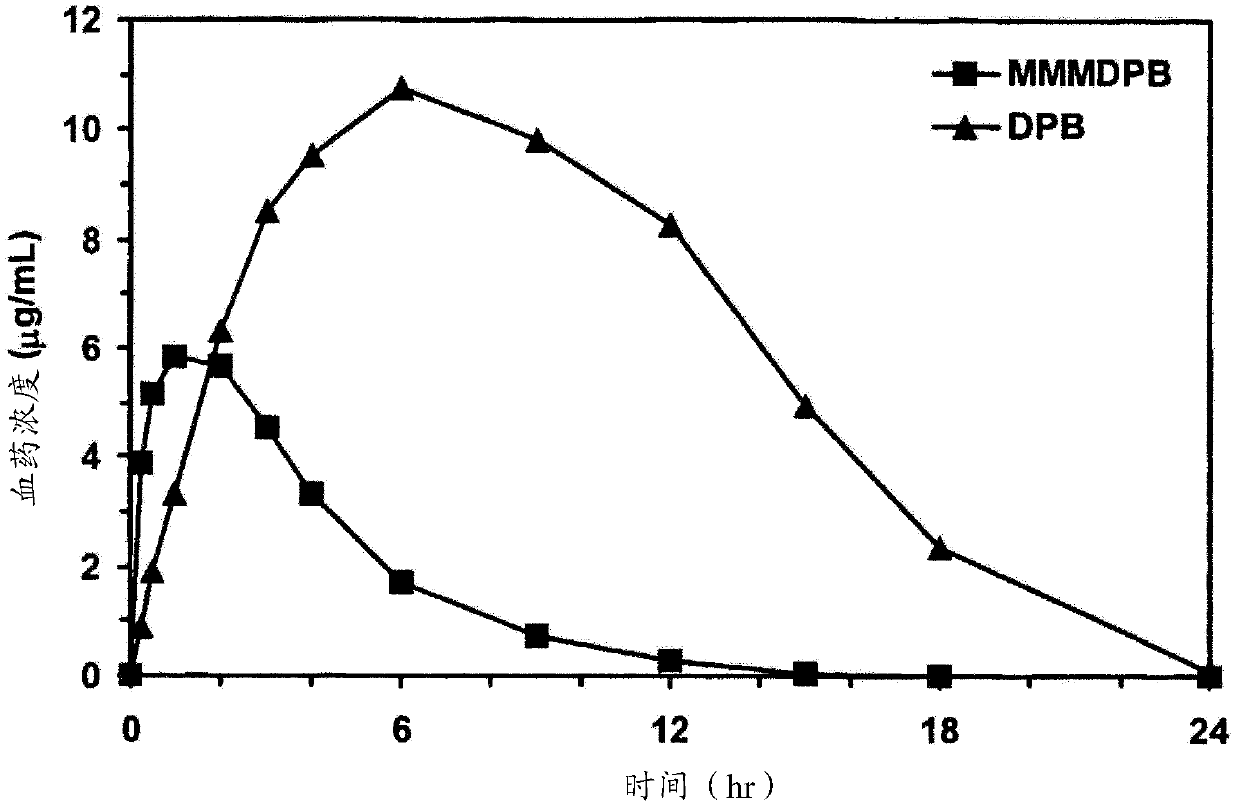
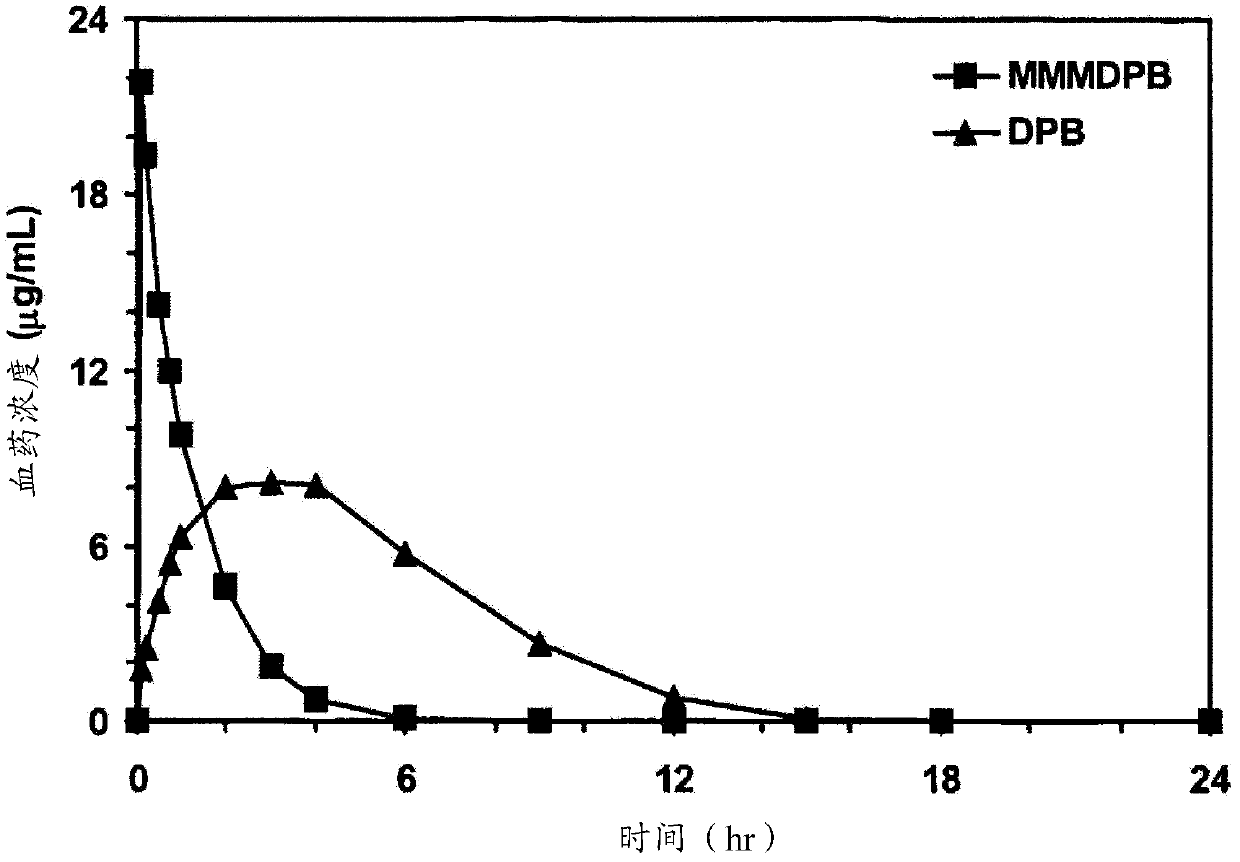
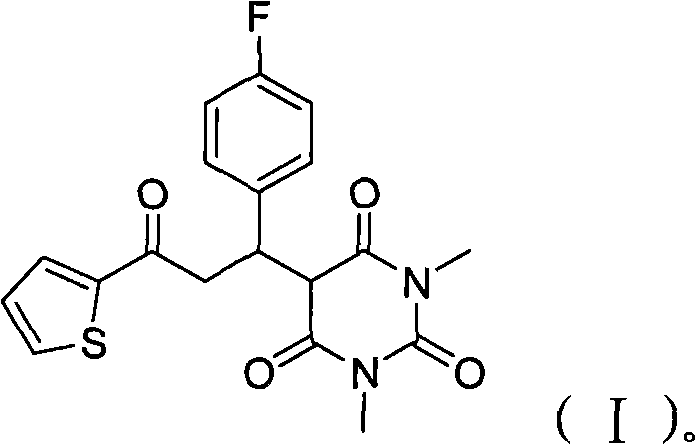
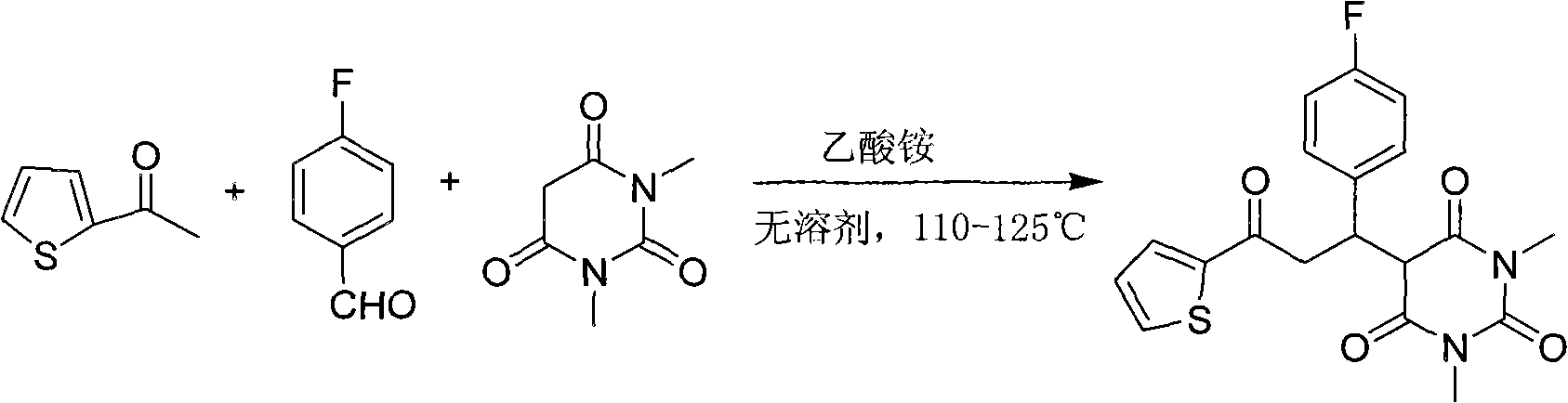
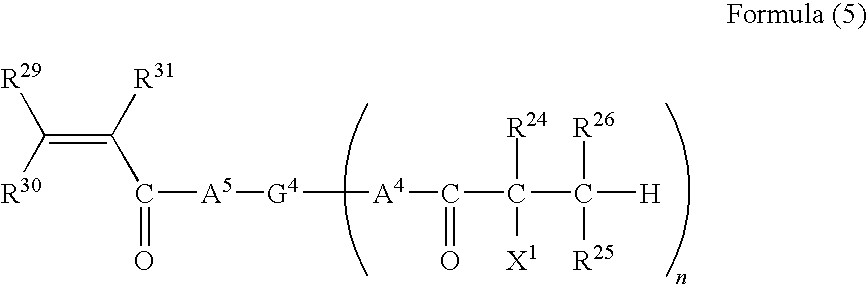Patents
Literature
36 results about "Barbituric acid derivative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Paste-like bone cement
ActiveUS20110313078A1High initial stabilityLow post-cureImpression capsSurgical adhesivesHeavy metal compoundCompound (substance)
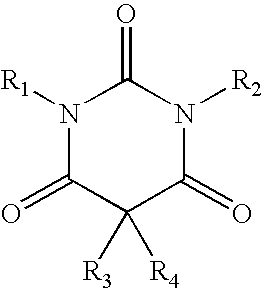
A kit is provided based on two pastes designed to produce bone cement with high initial stability and therefore low post-cure. The kit includes a paste A and a paste B, wherein paste A contains (a1) a polymerizable monomer having a pH in water in the range of 5-9, (a2) a filling agent insoluble in (a1), and (a3) a barbituric acid derivative selected from the group consisting of 1,5-disubstituted barbiturates, 1,3,5-trisubstituted barbiturates, and 1,3,5-tetrasubstituted barbiturates, and paste B contains (b1) a polymerizable monomer having a pH in water in the range of 5-9, (b2) a filling agent insoluble in (b1), (b3) a peroxide soluble in (b1), (b4) a heavy metal compound insoluble in (b1) and selected from the group consisting of heavy metal salts and heavy metal complexes, and wherein at least one of the pastes A and B contains a halide salt.
Owner:HERAEUS MEDICAL
Non-sedating barbituric acid derivatives
The present invention relates to novel non-sedating barbituric acid derivatives, pharmaceutical compositions containing them and methods of neuroprotection in cases of cerebral ischemia, head trauma and other acute neurologic injuries, and prevention of resulting neuronal damage. The invention also relates to the use of non-sedating barbituric acid derivatives given in a manner and dosage effective to produce blood levels and brain levels of these drugs and / or their active metabolites sufficient to provide a therapeutic effect.
Owner:TARO PHARMA INDS
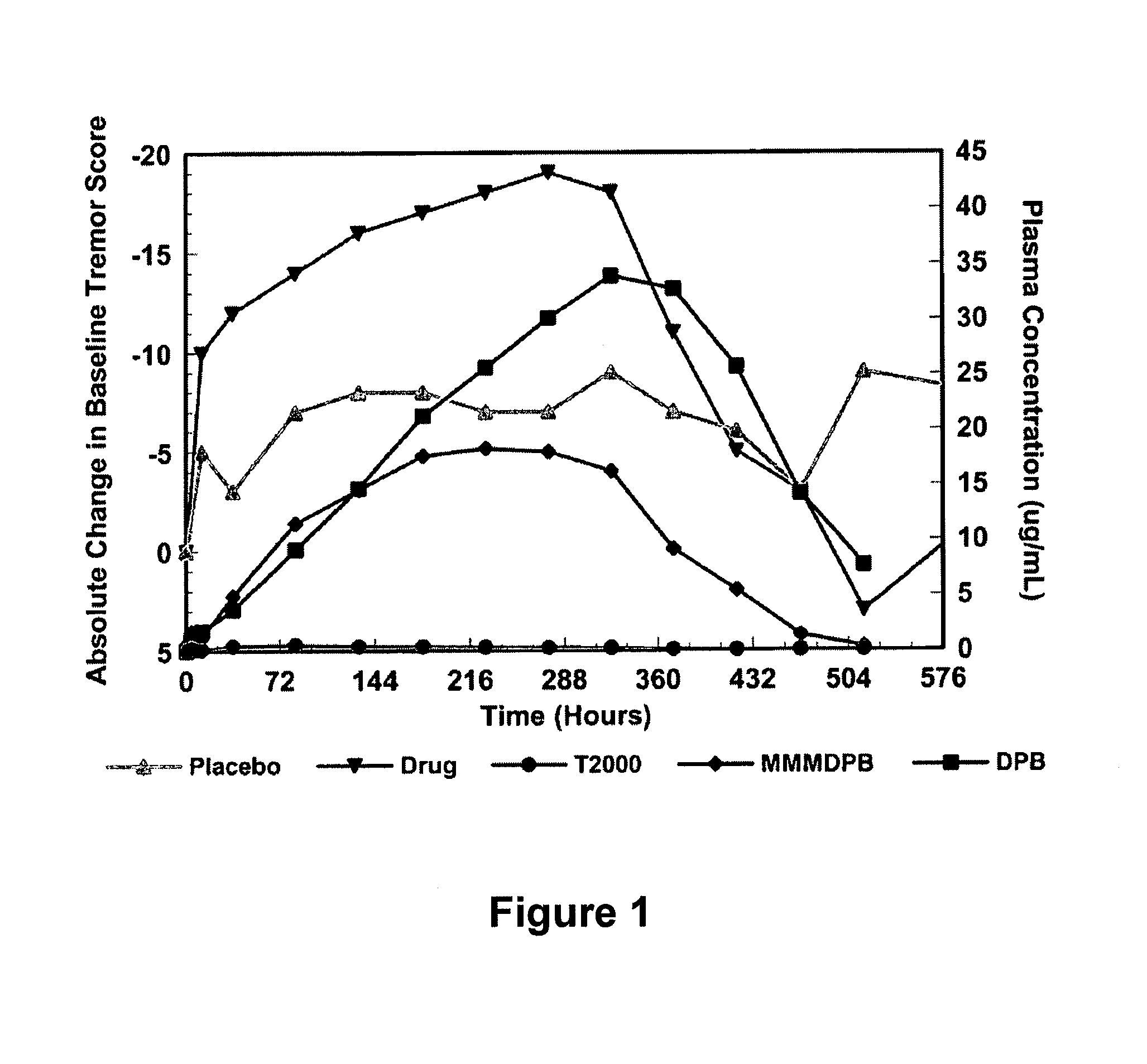
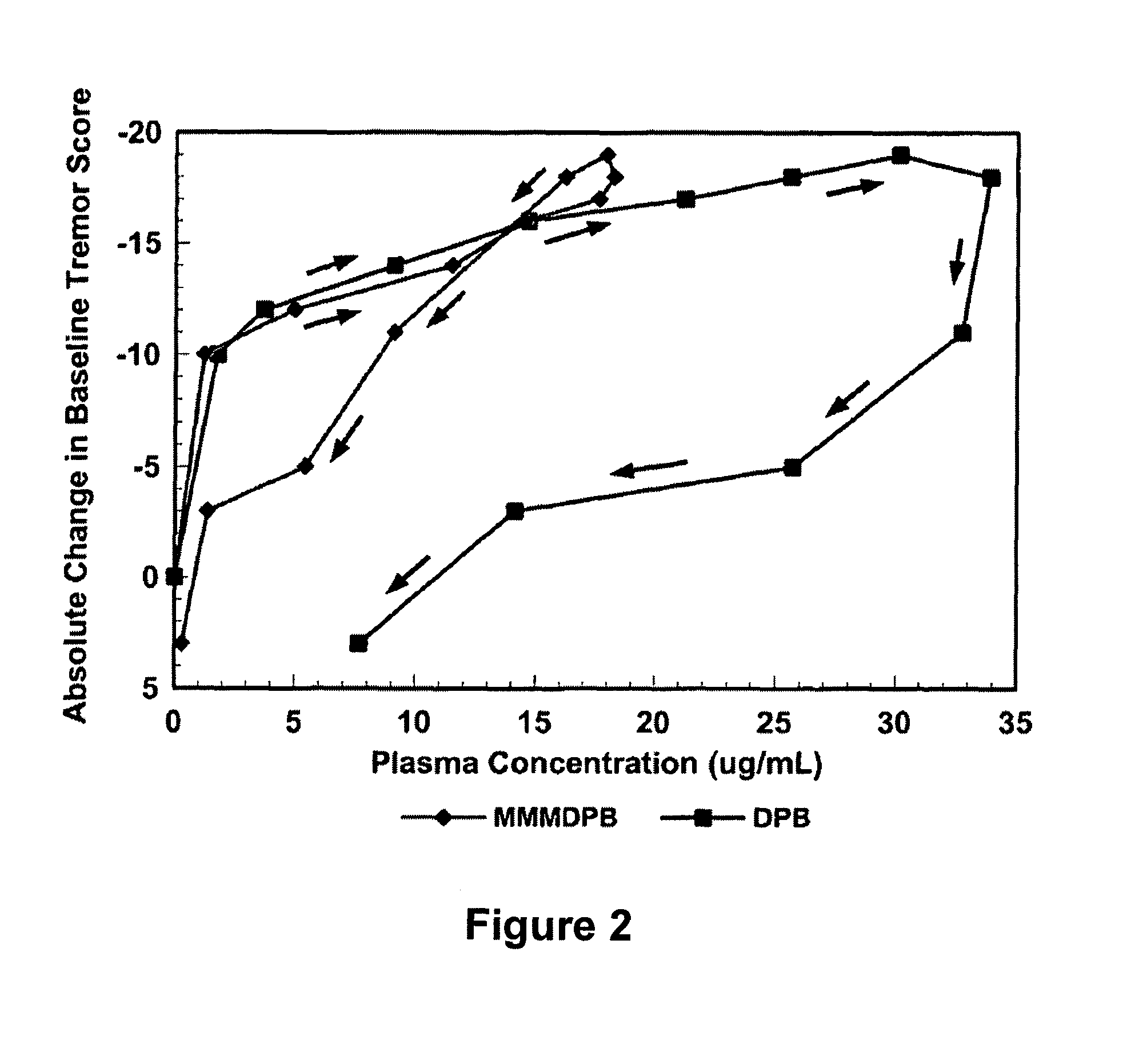
Method of treating movement disorders using barbituric acid derivatives
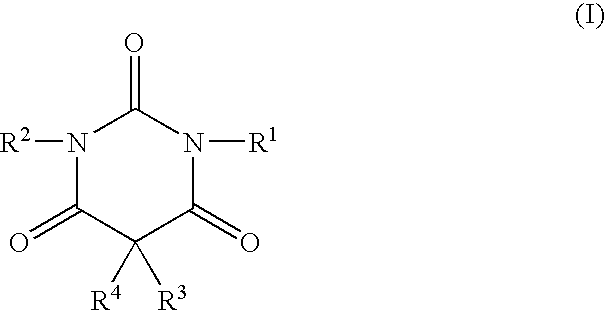
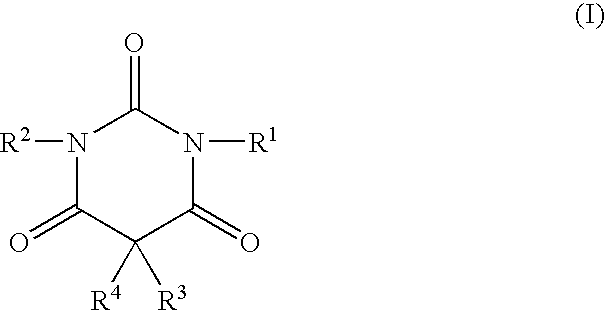
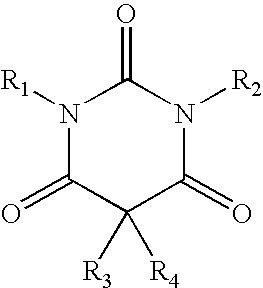
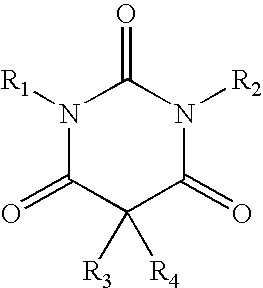
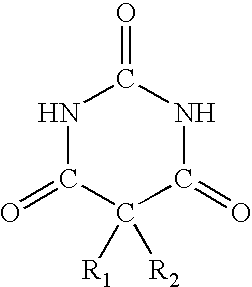
A method of treating movement disorders comprises administering to a human or animal subject in need of treatment a therapeutically effective amount of at least one compound according to the following formula:wherein R3 and R4 are each independently selected from the group consisting of lower alkyl, phenyl and lower alkyl substituted phenyl, and R1 and R2 are each independently either a hydrogen atom or a radical of the formulawherein R5 and R6 are each independently selected from the group consisting of H, lower alkyl, phenyl and lower alkyl substituted phenyl, its pharmaceutically acceptable salts, prodrugs, and metabolites thereof.
Owner:TARO PHARMA INDS
Non-sedating barbituric acid derivatives
The present invention relates to novel non-sedating barbituric acid derivatives, pharmaceutical compositions containing them and methods of neuroprotection in cases of cerebral ischemia, head trauma and other acute neurologic injuries, and prevention of resulting neuronal damage. The invention also relates to the use of non-sedating barbituric acid derivatives given in a manner and dosage effective to produce blood levels and brain levels of these drugs and / or their active metabolites sufficient to provide a therapeutic effect.
Owner:TARO PHARMA INDS
Composition and method for improved bioavailability and enhanced brain delivery of 5,5-diphenyl barbituric acid
InactiveUS20060122208A1Enhanced and efficient deliveryAdequate doseBiocideOrganic active ingredientsBioavailabilitySodium salt
The present invention relates to a composition and a method of delivering a barbituric acid derivative to the central nervous system of a mammal in need of treatment for neurological conditions. In particular, the present invention relates to a method of administering an oral dosage form of a sodium salt of 5,5-diphenyl barbituric acid to enhance the bioavailability of 5,5-diphenyl barbituric acid and brain delivery of same.
Owner:TARO PHARMA INDS
Method of treating movement disorders using barbituric acid derivatives
A method of treating movement disorders comprises administering to a human or animal subject in need of treatment a therapeutically effective amount of at least one compound according to the following formula: wherein R3 and R4 are each independently selected from the group consisting of lower alkyl, phenyl and lower alkyl substituted phenyl, and R1 and R2 are each independently either a hydrogen atom or a radical of the formula wherein R5 and R6 are each independently selected from the group consisting of H, lower alkyl, phenyl and lower alkyl substituted phenyl, its pharmaceutically acceptable salts, prodrugs, and metabolites thereof.
Owner:TARO PHARMA INDS
Photosensitive composition
InactiveUS6432613B1Improvement of resolution and adhesionReduce the amount requiredPhotosensitive materialsPhotomechanical apparatusResistAdduct
The present invention discloses a photo-sensitive composition, used as a solder resist or a photosensitive material for insulation layers in the production of printed circuit boards. The photo-sensitive composition comprises a prepolymer containing carboxylic groups and unsaturated vinyl groups; photoinitiator; unsaturated photo-monomer; and the reaction adduct of bismaleimide derivative, barbituric acid derivative and epoxy compounds. The obtained photosensitive composition exhibits high adhesion towards PI substrates, in addition, it can be developed with alkaline water. The photosensitive composition obtained in the invention is very useful in packaging substrates, such as P-BGA, T-BGA and F-CSP due to its high heat resistance and solder resistance.
Owner:IND TECH RES INST
N-hydroxylamino-barbituric acid derivatives
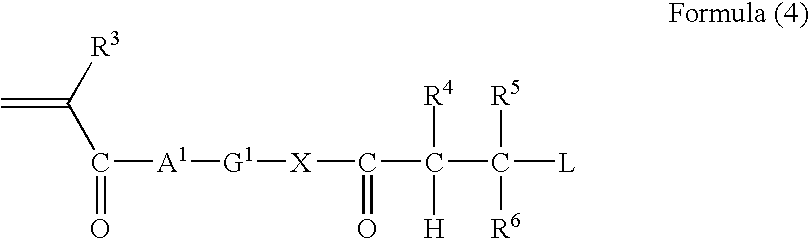
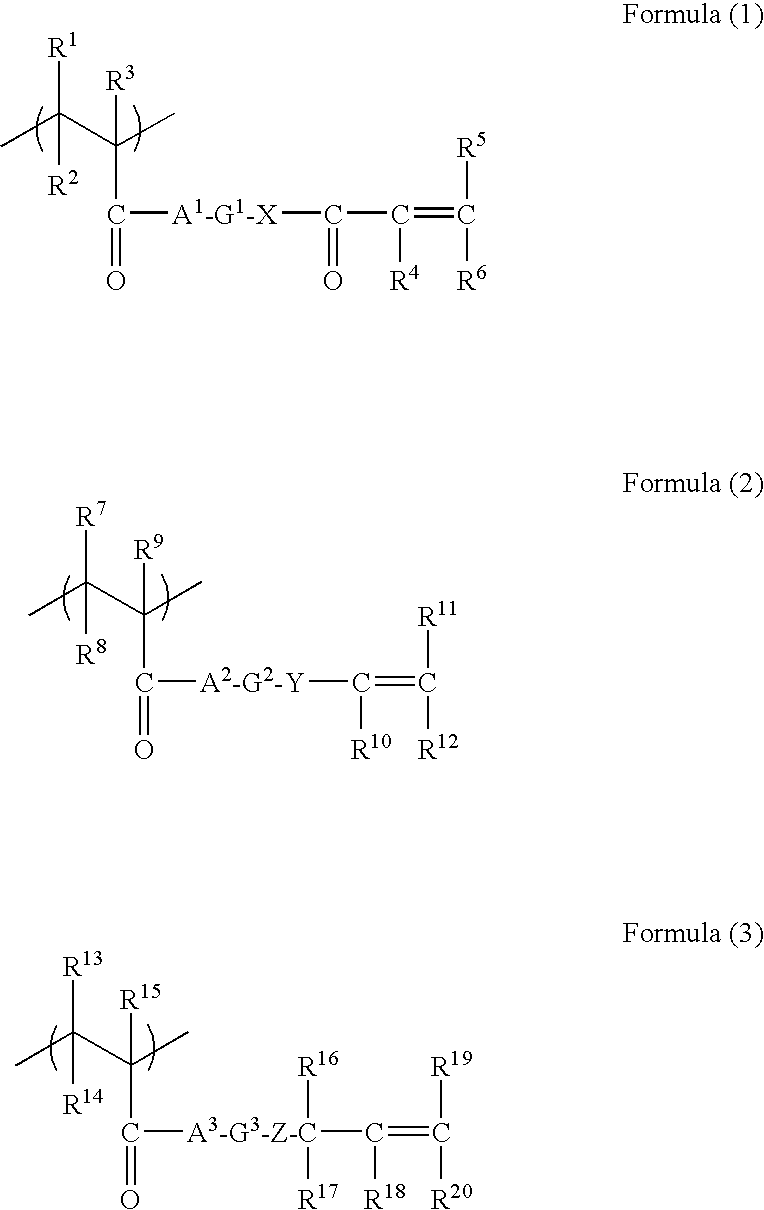
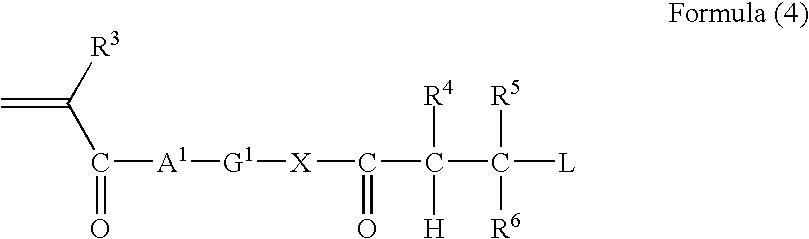
The present disclosure provides N-hydroxylamino-barbituric acid compounds of formulae (1)-(4), pharmaceutical compositions and kits comprising them, and methods of using such compounds or pharmaceutical compositions. The present disclosure provides methods of using such compounds or pharmaceutical compositions for treating heart failure.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Composition and method for improved bioavailability and enhanced brain delivery of 5,5-diphenyl barbituric acid
InactiveUS7683071B2Reduce deliveryAdequate doseOrganic active ingredientsBiocideBioavailabilitySodium salt
The present invention relates to a composition and a method of delivering a barbituric acid derivative to the central nervous system of a mammal in need of treatment for neurological conditions. In particular, the present invention relates to a method of administering an oral dosage form of a sodium salt of 5,5-diphenyl barbituric acid to enhance the bioavailability of 5,5-diphenyl barbituric acid and brain delivery of same.
Owner:TARO PHARMA INDS
Method of treating movement disorders using barbituric acid derivatives
A method of treating movement disorders comprises administering to a human or animal subject in need of treatment a therapeutically effective amount of at least one compound according to the following formula:wherein R3 and R4 are each independently selected from the group consisting of lower alkyl, phenyl and lower alkyl substituted phenyl, and R1 and R2 are each independently either a hydrogen atom or a radical of the formulawherein R5 and R6 are each independently selected from the group consisting of H, lower alkyl, phenyl and lower alkyl substituted phenyl, its pharmaceutically acceptable salts, prodrugs, and metabolites thereof.
Owner:TARO PHARMA INDS
Paste-like bone cement
ActiveUS8598251B2Improve stabilityLow post-cureImpression capsSurgical adhesivesHeavy metal compoundCompound (substance)
A kit is provided based on two pastes designed to produce bone cement with high initial stability and therefore low post-cure. The kit includes a paste A and a paste B, wherein paste A contains (a1) a polymerizable monomer having a pH in water in the range of 5-9, (a2) a filling agent insoluble in (a1), and (a3) a barbituric acid derivative selected from the group consisting of 1,5-disubstituted barbiturates, 1,3,5-trisubstituted barbiturates, and 1,3,5-tetrasubstituted barbiturates, and paste B contains (b1) a polymerizable monomer having a pH in water in the range of 5-9, (b2) a filling agent insoluble in (b1), (b3) a peroxide soluble in (b1), (b4) a heavy metal compound insoluble in (b1) and selected from the group consisting of heavy metal salts and heavy metal complexes, and wherein at least one of the pastes A and B contains a halide salt.
Owner:HERAEUS MEDICAL
Green curable composition, color filter and method of producing same
ActiveUS20090246649A1Excellent spectroscopic characteristicImprove finenessOptical filtersSolid-state devicesTotal solid contentPigment
Owner:FUJIFILM CORP
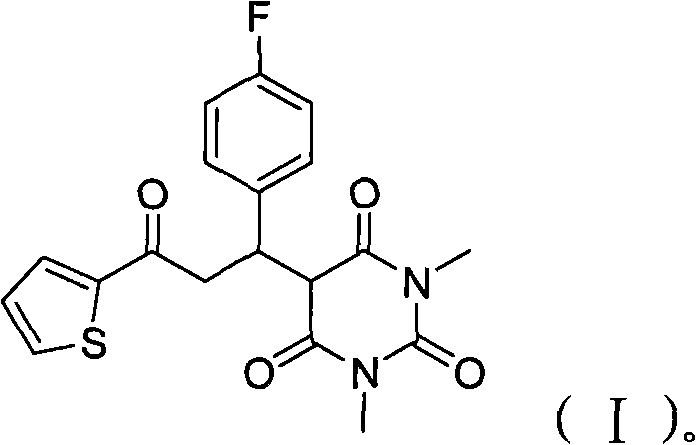
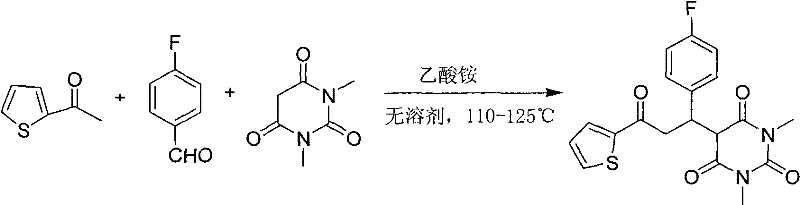
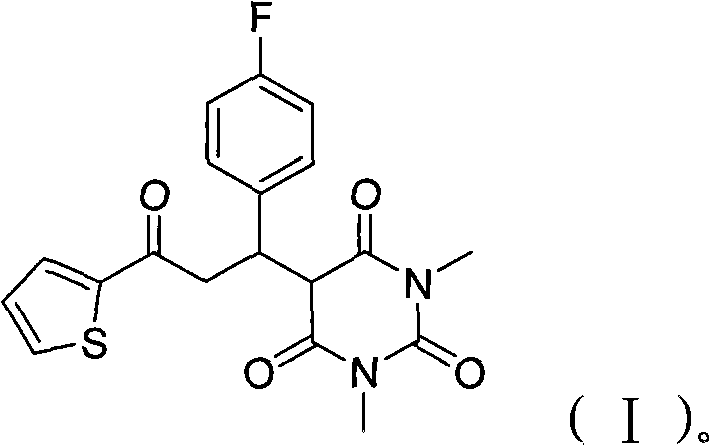
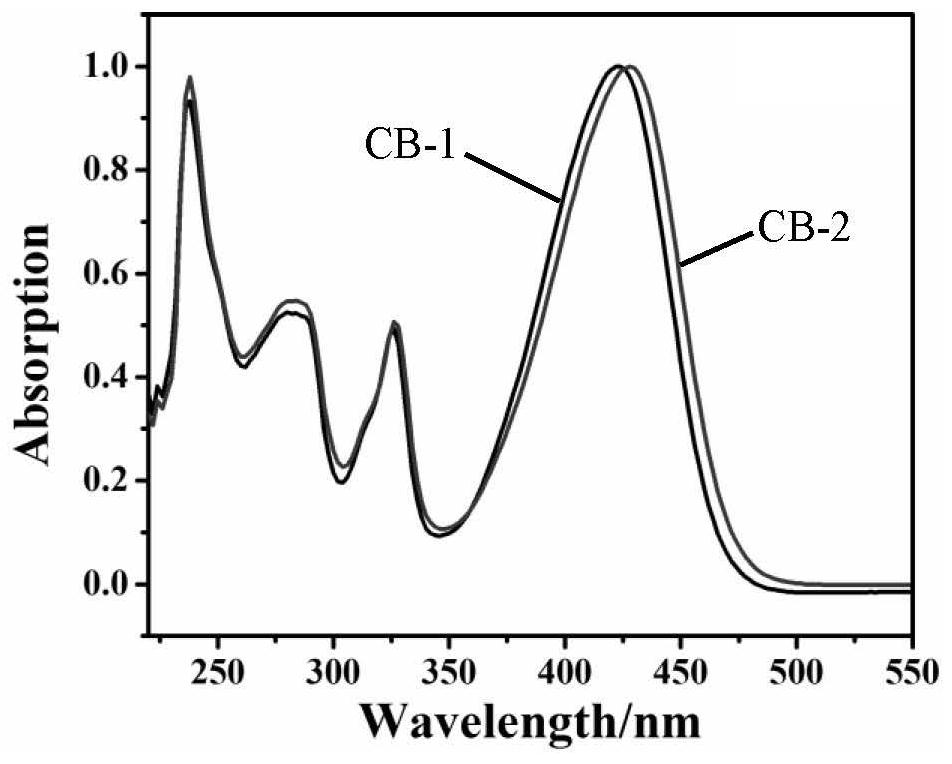
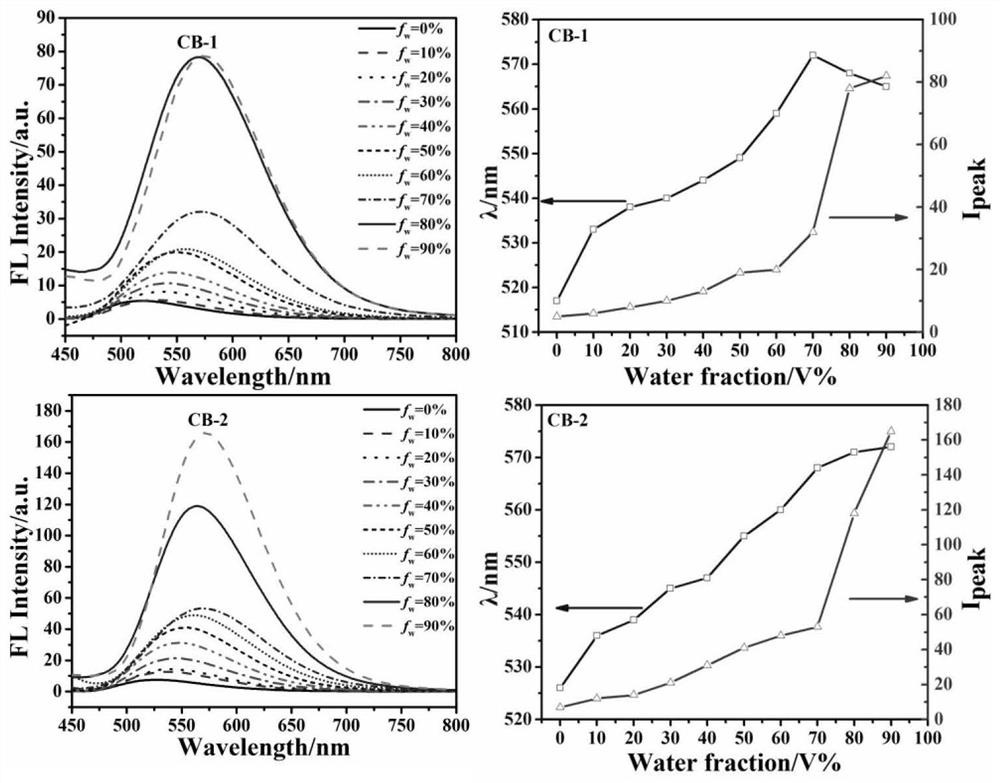
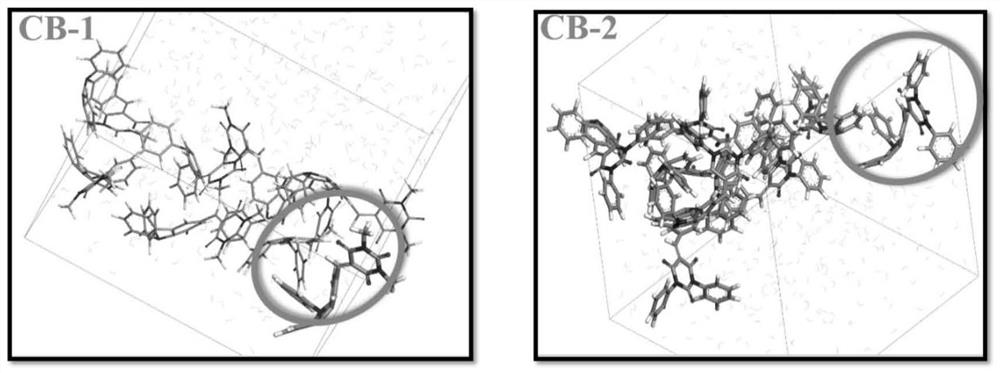
Barbituric acid derivative as well as preparation method and application thereof
ActiveCN110028456AHigh sensitivityGood choiceOrganic chemistryColor/spectral properties measurementsHydrogenBenzaldehyde
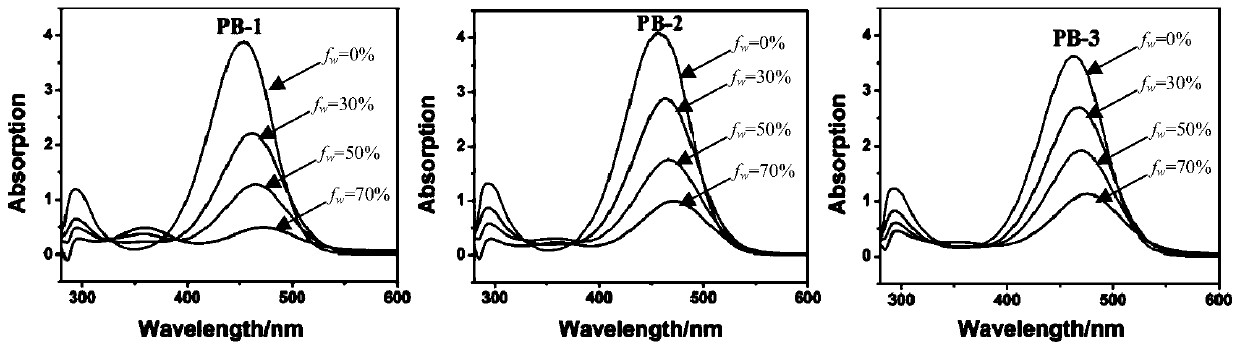
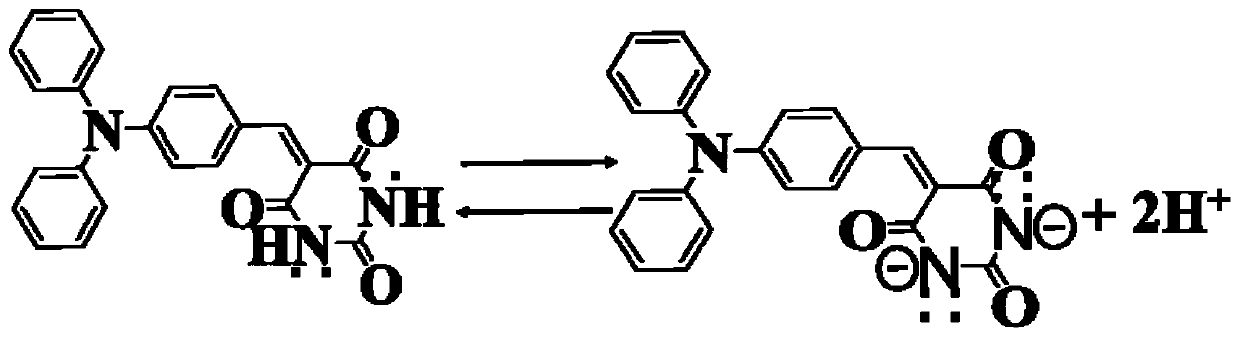
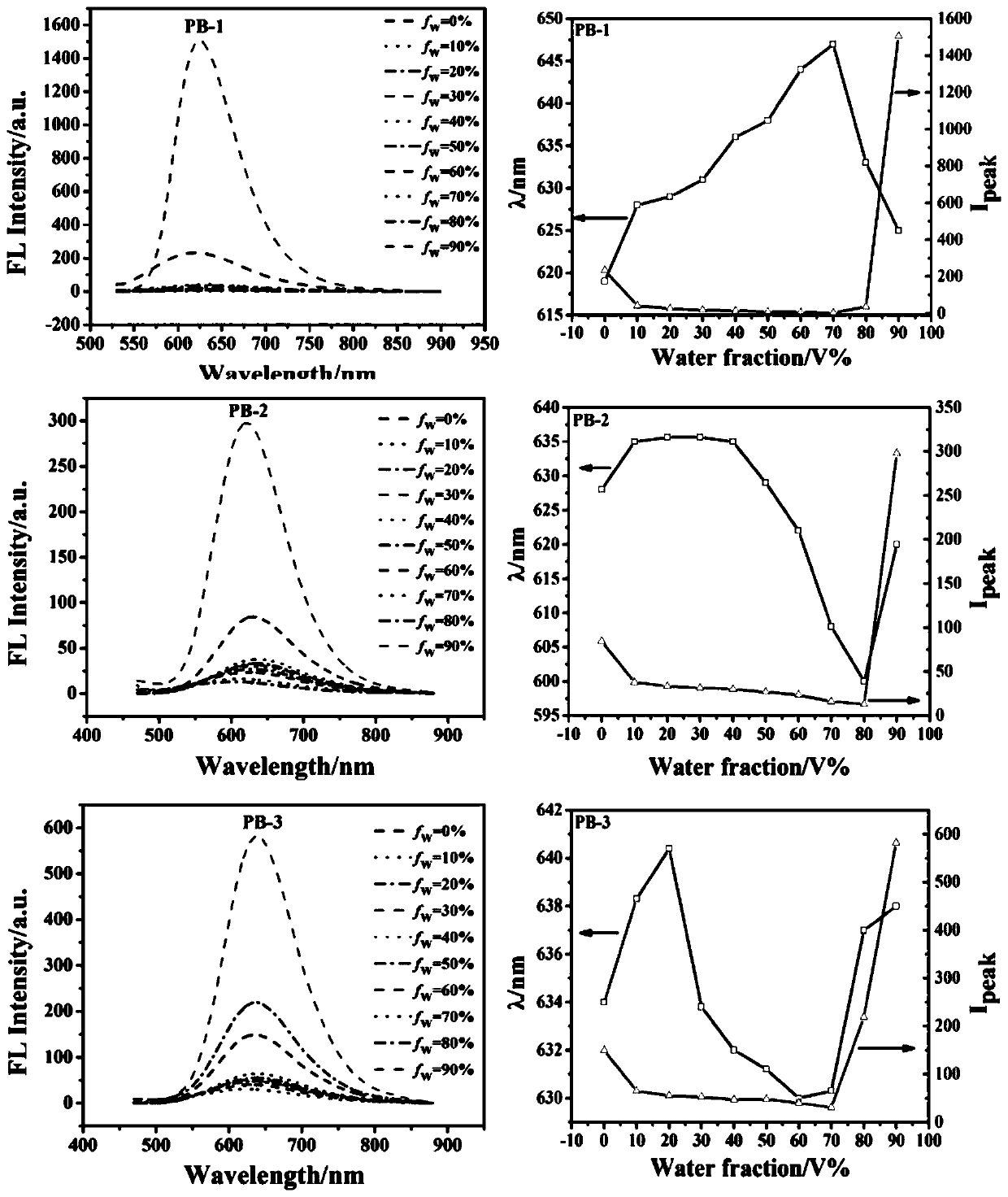
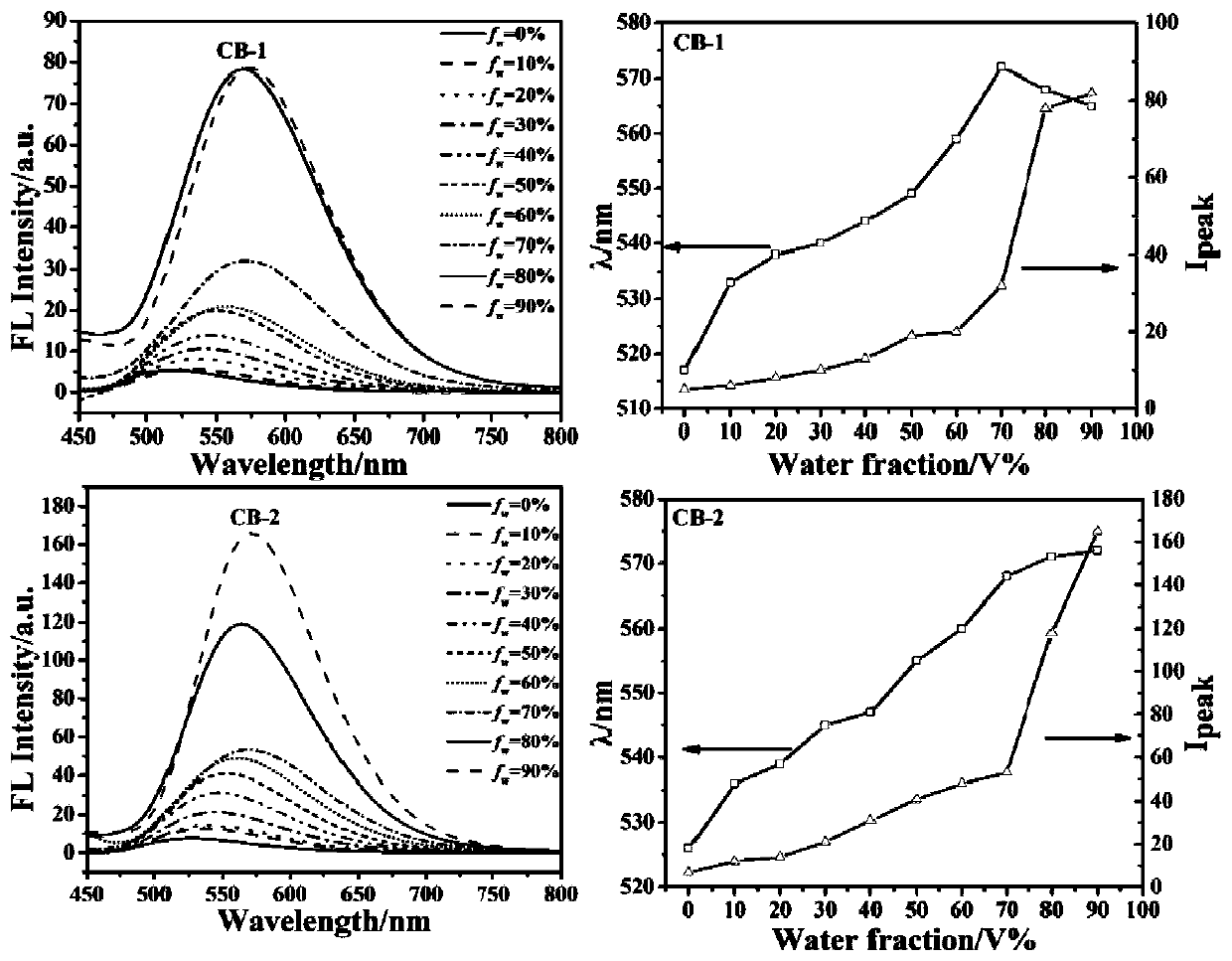
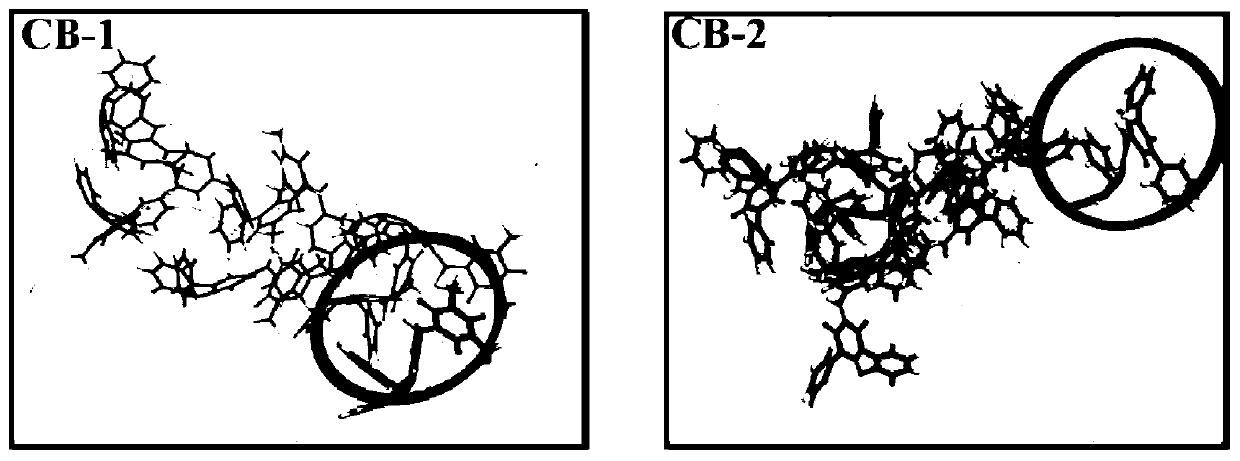
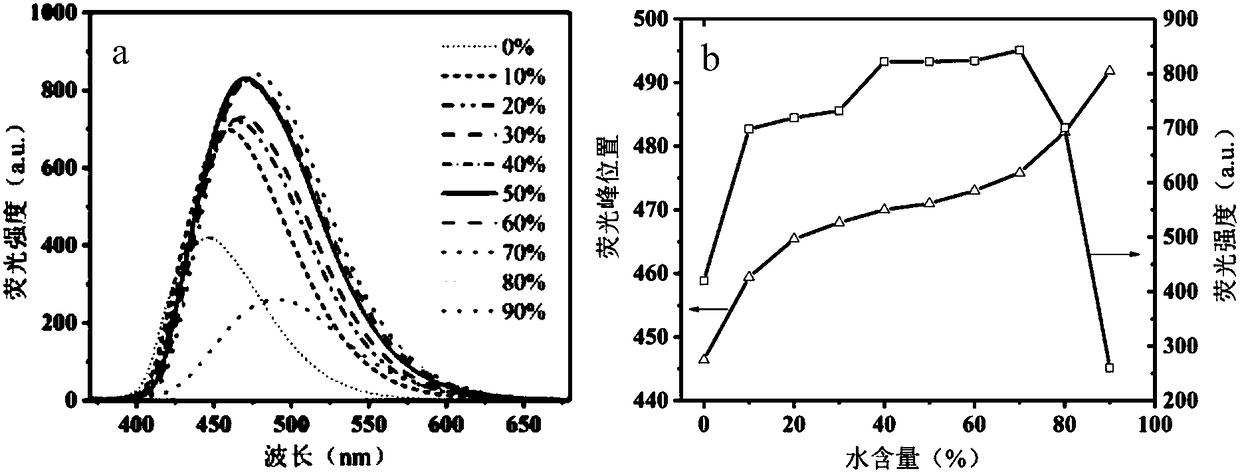
The invention provides a barbituric acid derivative as well as a preparation method and application thereof. The chemical structural formula is shown in the specification, and R is hydrogen, methyl orphenyl. The preparation method comprises the following steps: carrying out an aldol condensation reaction on 4-diphenylamino benzaldehyde and a barbituric acid compound to obtain a barbituric acid derivative, wherein the structural formula of the barbituric acid compound is shown in the specification, and R is hydrogen, methyl or phenyl. The barbituric acid derivative provided by the invention issimple in structure, and can generate aggregates under a high water content, so that the barbituric acid derivative can be used for detecting nitroaromatics in an aqueous medium; and the barbituric acid derivative has the advantages of high sensitivity, good selectivity, low detection limit and the like for detecting nitroaromatics.
Owner:QILU UNIV OF TECH
Spiro condensed barbituric acid derivatives for use as antibacterial
InactiveCN101687871AAntibacterial agentsOrganic active ingredientsAcid derivativeAntibacterial agent
Owner:ASTRAZENECA AB
Polyamide resin composition including carboxylic acid derivative
InactiveCN108368337AImprove the effect of crystallizationImprove heat resistanceHeat resistanceAcid derivative
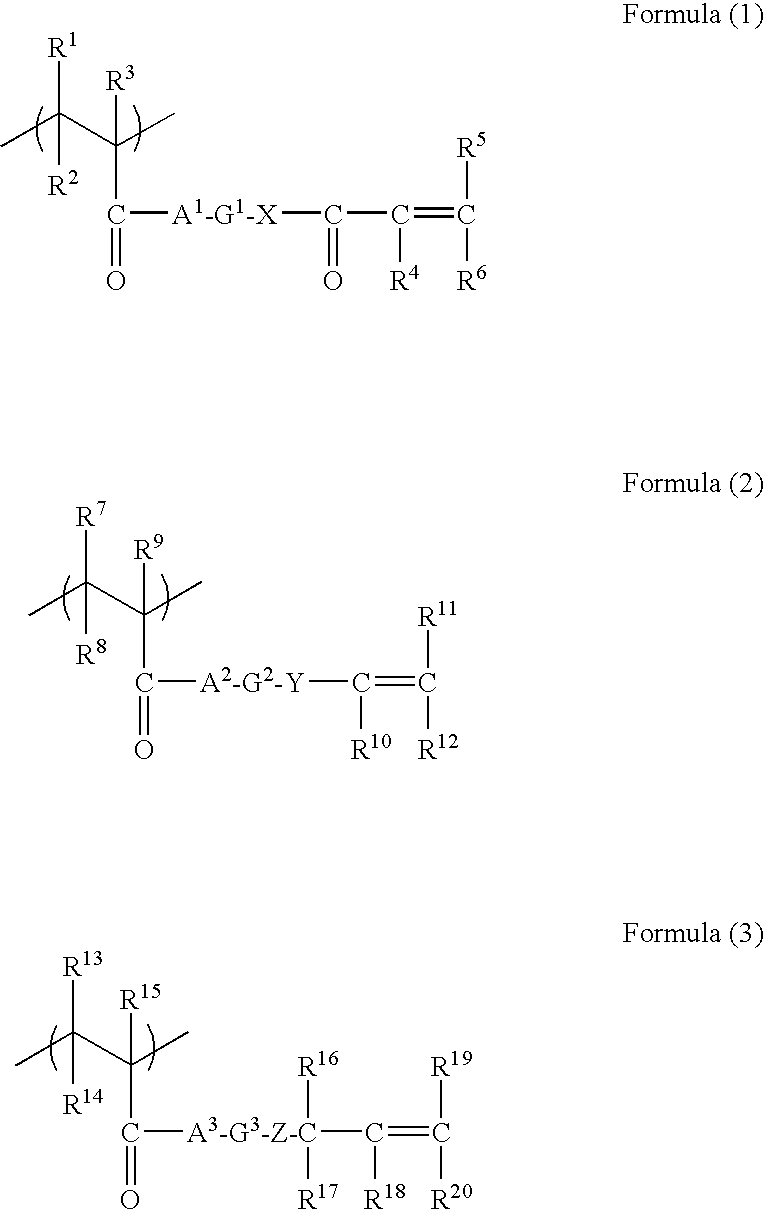
To provide a polyamide resin composition containing an added crystal nucleator suitable for promoting the crystallization of a polyamide resin and not triggering discoloration in the resin, the polyamide resin composition being capable of raising the crystallization rate and achieving better molding process properties and heat resistance in comparison with the polyamide resin. A polyamide resin composition including a polyamide resin and a crystal nucleator comprising a carboxylic acid derivative represented by formula [1]. B1-L1-A-L2-B2 [1] (In the formula, A represents an optionally substituted C1-6 alkylene group or C6-10 divalent aromatic group, B1 and B2 represent optionally substituted C3-6 cycloalkyl groups or optionally substituted C6-10 aromatic groups, and L1 and L2 represent -C(=O)NR1- or -C(=O)O-.).
Owner:NISSAN CHEM CORP
Non-sedating barbituric acid derivatives
The present invention relates to novel non-sedating barbituric acid derivatives, pharmaceutical compositions containing them and methods of neuroprotection in cases of cerebral ischemia, head trauma and other acute neurologic injuries, and prevention of resulting neuronal damage. The invention also relates to the use of non-sedating barbituric acid derivatives given in a manner and dosage effective to produce blood levels and brain levels of these drugs and / or their active metabolites sufficient to provide a therapeutic effect.
Owner:TARO PHARMA INDS
Composition and method for improving bioavailability and enhancing brain delivery of 5,5-diphenyl barbituric acid
InactiveCN101052403AImprove complianceImprove reliabilityOrganic active ingredientsSenses disorderDiseaseBioavailability
The present invention relates to a composition and a method of delivering a barbituric acid derivative to the central nervous system of a mammal in need of treatment for neurological conditions. In particular, the present invention relates to a method of administering an oral dosage form of a sodium salt of 5,5-diphenyl barburtic acid to enhance the bioavailability of 5,5-diphenyl barbituric acid and brain delivery of same.
Owner:TARO PHARMA INDS
Polymer and lithium battery
PendingCN112300382AIncrease capacitanceImprove battery efficiencyNegative electrodesLi-accumulatorsPolymer scienceThiourea
Owner:王复民
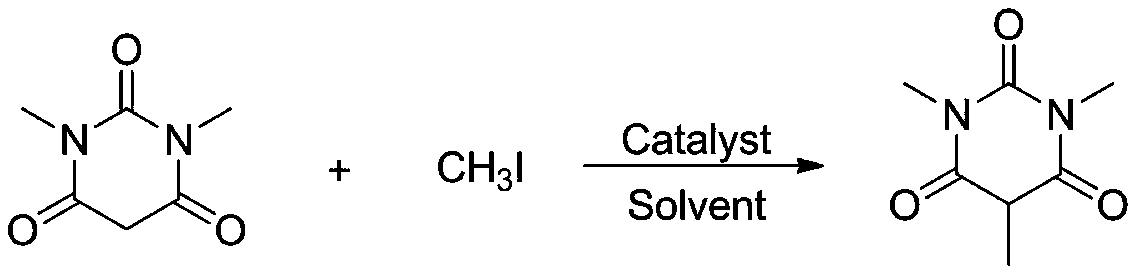
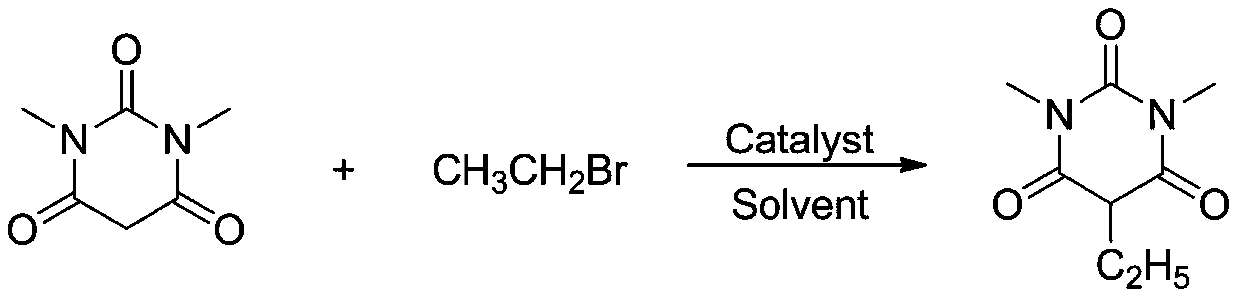
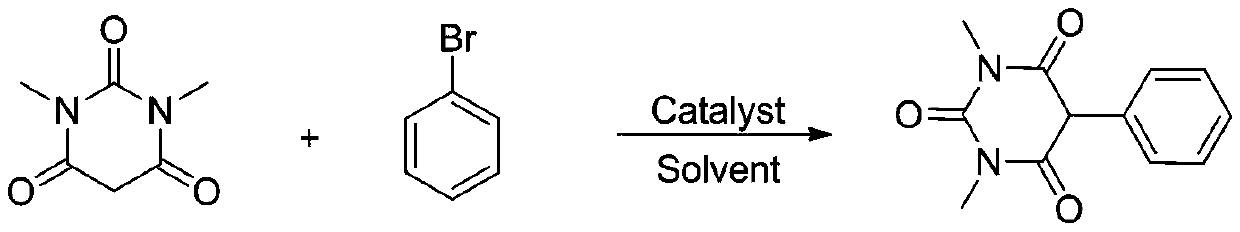
Method for synthesizing 5-substituted barbituric acid derivative under catalysis of rare earth chloride
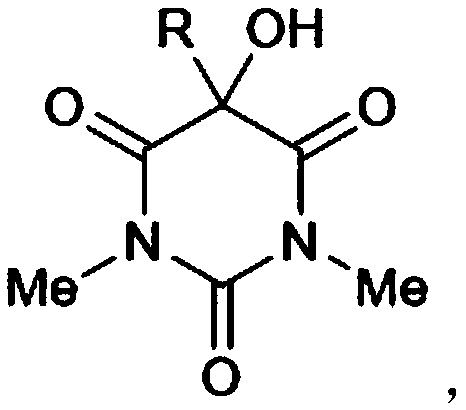
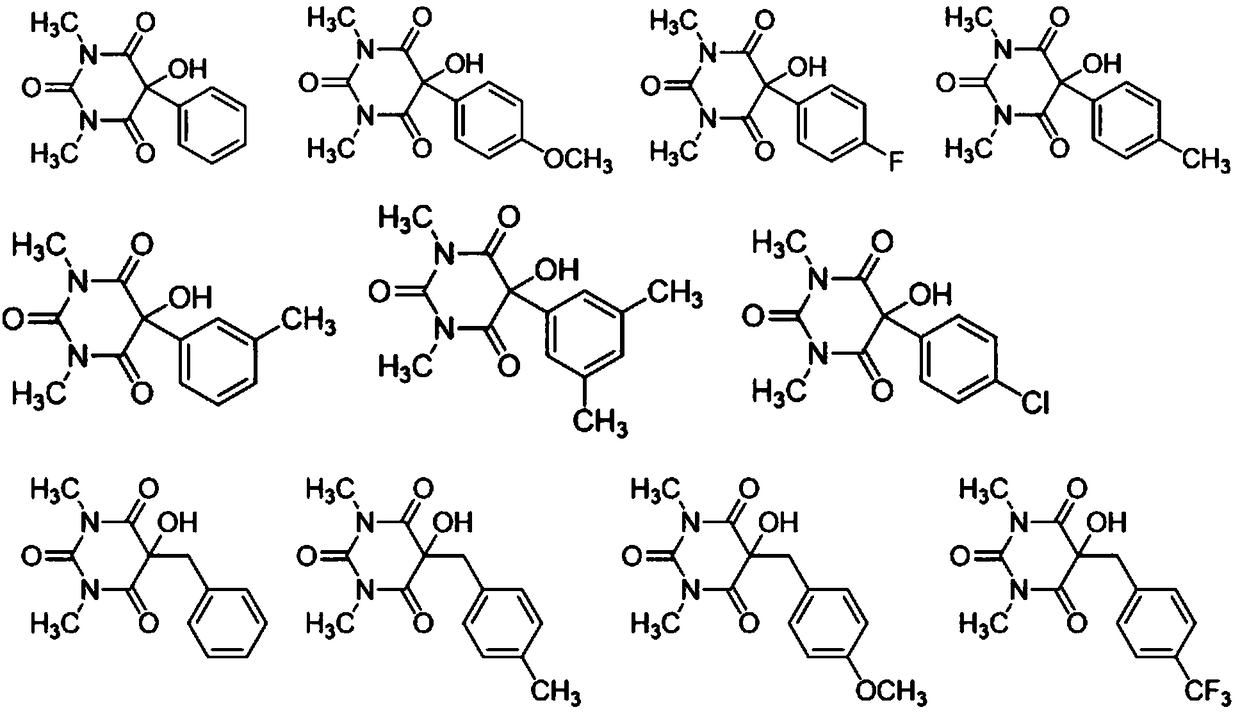
The invention belongs to the technical field of synthetic chemistry, and particularly relates to a method for synthesizing a 5-substituted barbituric acid derivative under the catalysis of a rare earth chloride. The preparation method comprises: dissolving a halogenated hydrocarbon and 1,3-dimethyl barbituric acid in an organic solvent, carrying out a reaction for 6-10 h at a room temperature by using a rare earth chloride as a catalyst, and separating and purifying to obtain the 5-substituted barbituric acid derivative. According to the invention, the method has characteristics of simple andenvironmentally-friendly synthesis process, excellent selectivity, high yield and wide substrate range, and further has wide application value in the fields of biology, pharmaceutical chemistry industry and the like.
Owner:SHANGHAI URBAN CONSTR VOCATIONAL COLLEGE
Barbituric acid derivative, preparation method thereof, and application of derivative in data encryption and decryption
ActiveCN110642840AGood fluorescence activityCrystallization-induced emission enhancementOrganic chemistryInksCombinatorial chemistryEncryption decryption
The invention provides a barbituric acid derivative, a preparation method thereof, and an application of the derivative in data encryption and decryption. The chemical structure of the barbituric acidderivative is represented by formula I shown in the description. The barbituric acid derivative has the characteristic of crystallization-induced emission enhancement, and can be used for data encryption and decryption.
Owner:QILU UNIV OF TECH
Method for greenly synthesizing 5-hydroxy-5-alkyl disubstituted barbituric acid derivative by amine catalysis of air oxidation
InactiveCN108912057AOxidation reaction reaction conditions are mildEasy to controlOrganic chemistryArylMetal catalyst
The invention relates to the field of oxidative synthesis, and in particular, relates to a 5-hydroxy-5-alkyl disubstituted barbituric acid derivative greenly synthesized by amine catalysis of air oxidation and a method thereof. The amine-catalyzed oxidation reaction of a 5-substituted 1,3-dimethylbarbituric acid derivative is studied; reaction catalysts and solvents are screened, hydroxylation ofalpha-C-H of 5-aryl or benzyl substituted barbituric acid compounds is found out to be realized through catalytic reaction under different conditions. The method has the following characteristics: (1)air is used as the source of hydroxyl functional groups, so the requirements of green development are met; (2) easily available and cheap alkali R3N rather than expensive metal catalysts is used; and(3) stoichiometric harmful phosphine compounds are prevented from being used as additives and reductants.
Owner:HENAN UNIVERSITY
Barbituric acid derivatives and preparation method thereof
InactiveCN102070623BIncrease fat solubilityImprove hydrophobicityOrganic chemistrySolubilityBond energy
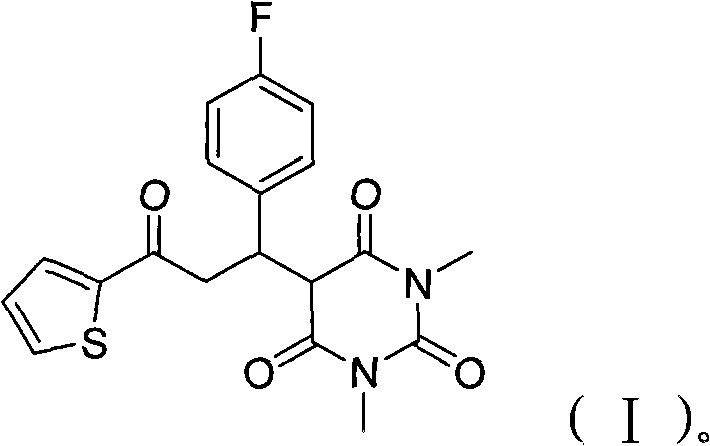
The invention provides barbituric acid derivatives and a preparation method thereof. The barbituric acid derivatives provided by the invention introduce fluorine atoms which have small radiuses and high electronegativity, and the bond energy of formed C-F bonds is higher than that of C-H bonds, thereby improving the stability and bioactivity of organic fluorine compounds; and the organic fluorinecompounds have relatively higher lipid solubility and relatively higher hydrophobicity. The barbituric acid derivatives provided by the invention introduce thiophene at the same time of introducing the fluorine atoms so as to achieve higher potential bioactivity and be easier to absorb. In-vitro antibacterial tests show that the compounds have a relatively better inhibiting effect on tested bacteria, and can be further developed as antibacterial agents or leading compounds. In the preparation method provided by the invention, a solvent-free one-step synthesis method is adopted; and the preparation method has the advantages of readily-available raw materials, simple operations, environmental-friendliness, high yield and the like.
Owner:XINXIANG MEDICAL UNIV
Barbituric acid derivatives and preparation methods and their application in data encryption and decryption
ActiveCN110642840BGood fluorescence activityCrystallization-induced emission enhancementOrganic chemistryInksCombinatorial chemistryEncryption decryption
The invention provides a barbituric acid derivative, a preparation method thereof, and an application of the derivative in data encryption and decryption. The chemical structure of the barbituric acidderivative is represented by formula I shown in the description. The barbituric acid derivative has the characteristic of crystallization-induced emission enhancement, and can be used for data encryption and decryption.
Owner:QILU UNIV OF TECH
Composition and method for enhanced delivery of 5,5-diphenyl barbituric acid
The present invention relates to a composition and a method of delivering a barbituric acid derivative to the central nervous system of a mammal in need of treatment for neurological conditions. In particular, the present invention relates to a method of administering an oral dosage form of a sodium salt of 5,5-diphenyl barburtic acid to enhance the bioavailability of 5,5-diphenyl barbituric acid and brain delivery of same.
Owner:TARO PHARMA INDS
Barbituric acid derivatives and preparation method thereof
InactiveCN102070623AIncrease fat solubilityImprove hydrophobicityOrganic chemistryBond energySolubility
The invention provides barbituric acid derivatives and a preparation method thereof. The barbituric acid derivatives provided by the invention introduce fluorine atoms which have small radiuses and high electronegativity, and the bond energy of formed C-F bonds is higher than that of C-H bonds, thereby improving the stability and bioactivity of organic fluorine compounds; and the organic fluorine compounds have relatively higher lipid solubility and relatively higher hydrophobicity. The barbituric acid derivatives provided by the invention introduce thiophene at the same time of introducing the fluorine atoms so as to achieve higher potential bioactivity and be easier to absorb. In-vitro antibacterial tests show that the compounds have a relatively better inhibiting effect on tested bacteria, and can be further developed as antibacterial agents or leading compounds. In the preparation method provided by the invention, a solvent-free one-step synthesis method is adopted; and the preparation method has the advantages of readily-available raw materials, simple operations, environmental-friendliness, high yield and the like.
Owner:XINXIANG MEDICAL UNIV
Barbituric acid derivative of disubstituted thiophene and preparation method thereof
ActiveCN108484588AImprove solubilityAggregation-induced luminescenceOrganic chemistryTenebresent compositionsSolid-stateBarbituric acid derivative
The invention discloses a barbituric acid derivative of disubstituted thiophene and a preparation method thereof. The chemical structural formula of the barbituric acid derivative is as shown in the description. According to the invention, the thiophene-based barbituric acid derivative is synthesized for the first time, experiments prove that the derivative has higher solid-state luminescence efficiency and obvious color contrast.
Owner:QILU UNIV OF TECH
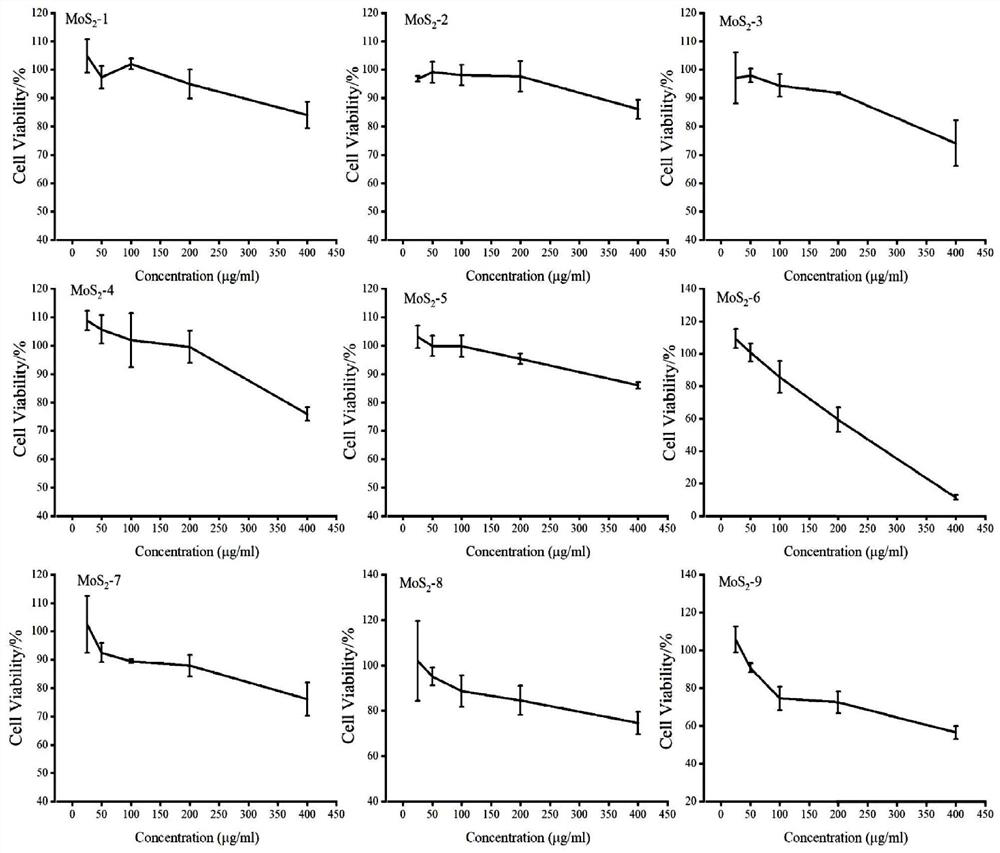
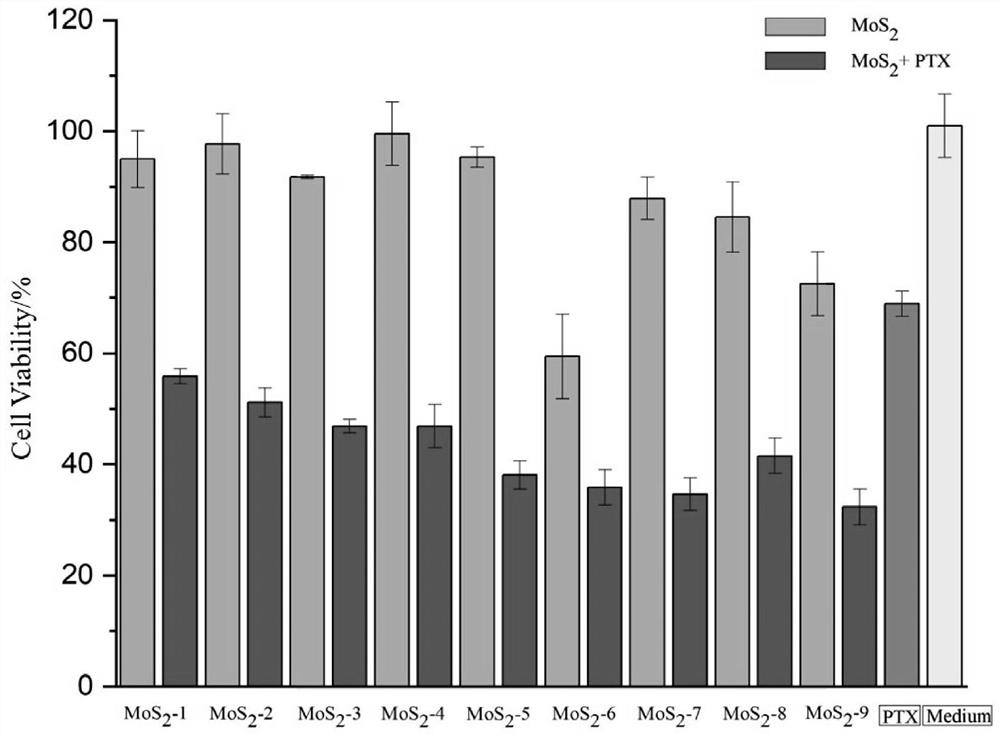
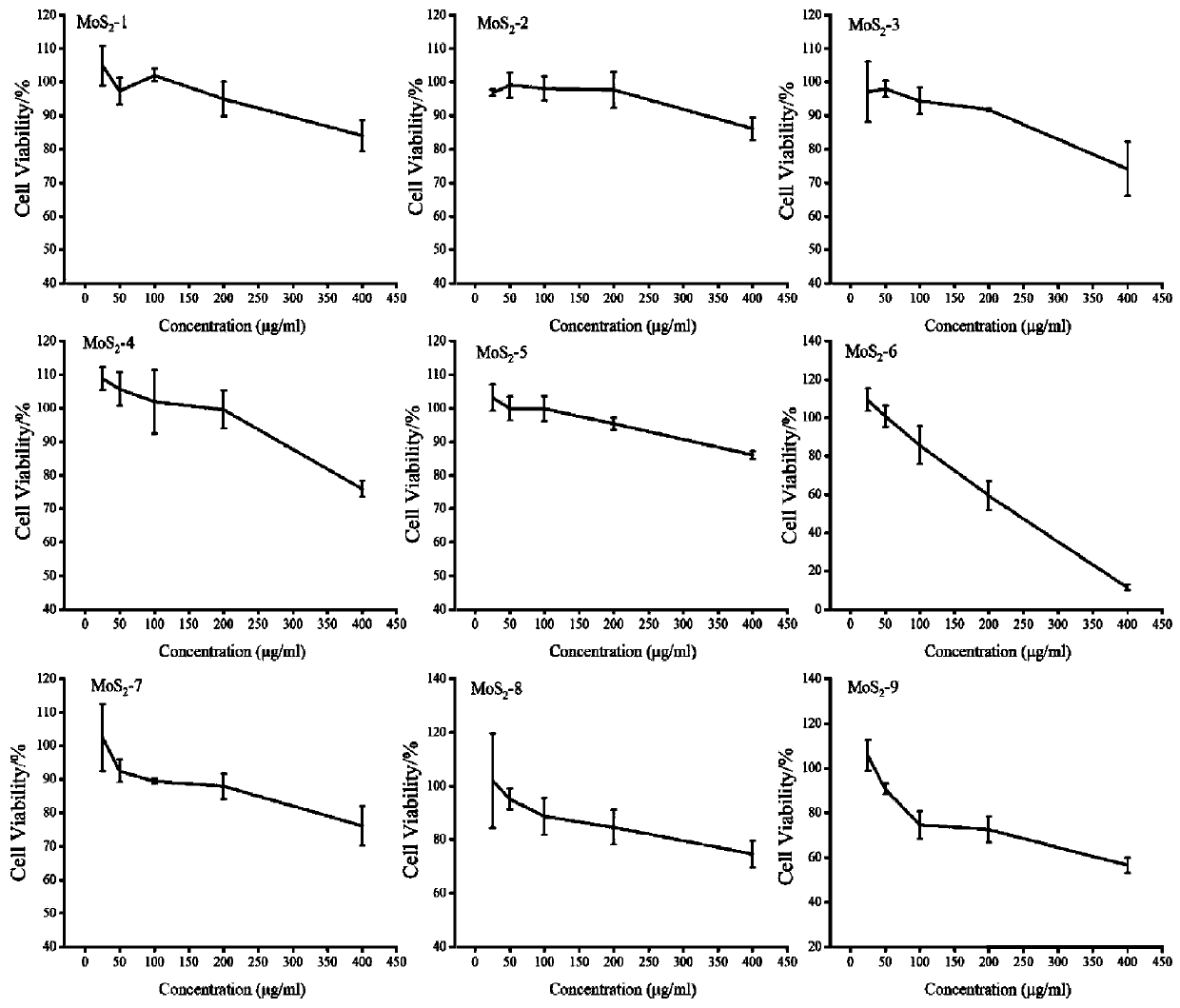
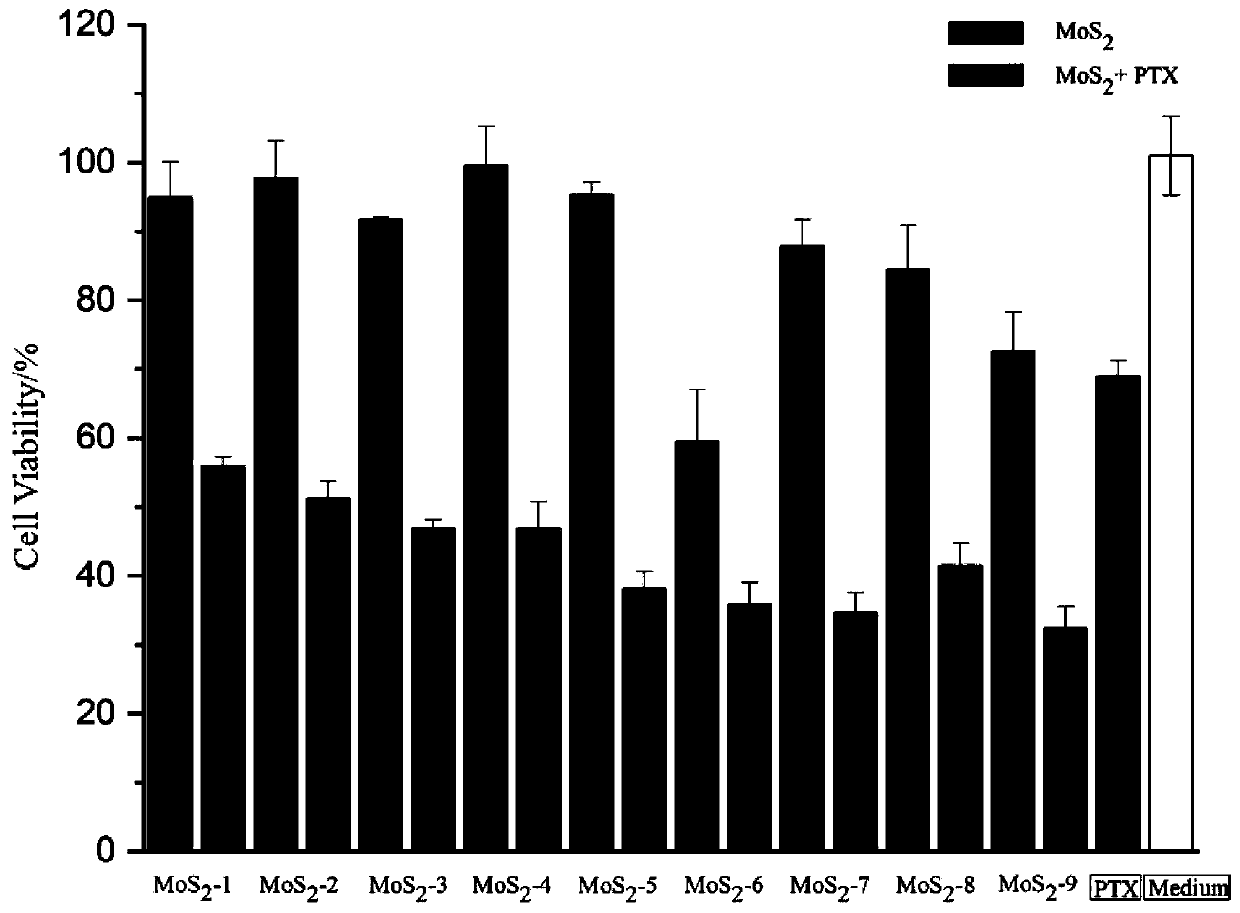
Molybdenum disulfide two-dimensional nanomaterials modified by barbituric acid derivatives and their applications
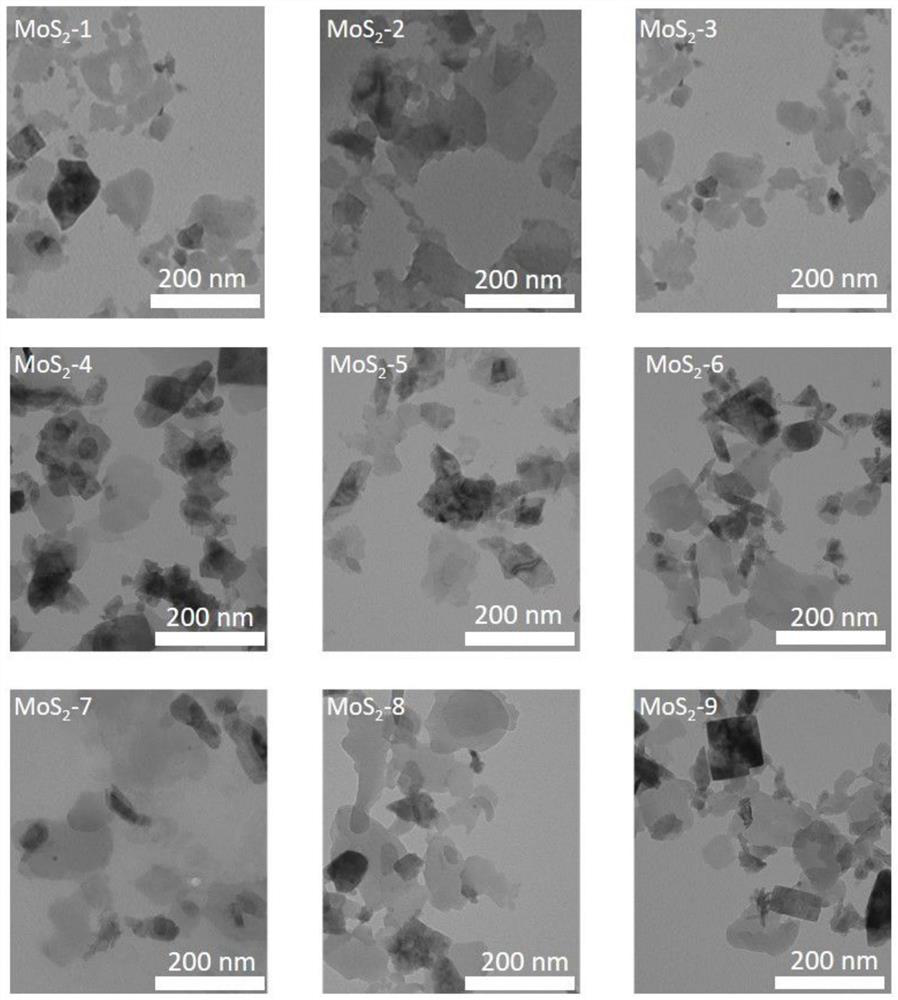
ActiveCN110859962BEliminate irritationReduce concentrationOrganic active ingredientsInorganic non-active ingredientsThio-Thiobarbituric acid
The invention discloses a thiobarbituric acid derivative molecule modified molybdenum disulfide two-dimensional nano material with diversified hydrophilic and hydrophobic properties and charges, whichis a molybdenum disulfide nano material respectively modified by Ligand 1-Ligand 9 ligand molecular compounds shown as formulas (I) to (IX), and is sequentially named as MoS2 <-1>-MoS2 <-9>. The invention also discloses an application of the molybdenum disulfide nano material in preparation of drugs and drug carriers for inhibiting tumor cell growth, or in preparation of paclitaxel-loaded drug complexes for inhibiting tumor cell growth as a drug carrier. The experiments prove that the thiobarbituric acid derivative molecule modified molybdenum disulfide two-dimensional nano material with diversified hydrophilic and hydrophobic properties and charges is expected to become a potential drug or drug carrier for tumor synergistic treatment, and has broad scientific research and clinical application prospects.
Owner:SHANDONG UNIV
Barbituric acid derivative modified molybdenum disulfide two-dimensional nano material and application thereof
ActiveCN110859962AEliminate irritationReduce concentrationOrganic active ingredientsInorganic non-active ingredientsThio-Thiobarbituric acid
The invention discloses a thiobarbituric acid derivative molecule modified molybdenum disulfide two-dimensional nano material with diversified hydrophilic and hydrophobic properties and charges, whichis a molybdenum disulfide nano material respectively modified by Ligand 1-Ligand 9 ligand molecular compounds shown as formulas (I) to (IX), and is sequentially named as MoS2 <-1>-MoS2 <-9>. The invention also discloses an application of the molybdenum disulfide nano material in preparation of drugs and drug carriers for inhibiting tumor cell growth, or in preparation of paclitaxel-loaded drug complexes for inhibiting tumor cell growth as a drug carrier. The experiments prove that the thiobarbituric acid derivative molecule modified molybdenum disulfide two-dimensional nano material with diversified hydrophilic and hydrophobic properties and charges is expected to become a potential drug or drug carrier for tumor synergistic treatment, and has broad scientific research and clinical application prospects.
Owner:SHANDONG UNIV
Green curable composition, color filter and method of producing same
The invention provides a green curable composition including a colorant in an amount of 47.5% by mass or more with respect to the total solid content of the green curable composition, the colorant containing a halogenated copper phthalocyanine pigment and a barbituric acid derivative yellow pigment at a ratio of from 6 / 4 to 3 / 7 (halogenated copper phthalocyanine pigment / barbituric acid derivative yellow pigment); a colored pattern formed from the green curable composition; a color filter including the colored pattern; a solid-state image sensor including the color filter; and a method of producing the color filter.
Owner:FUJIFILM CORP
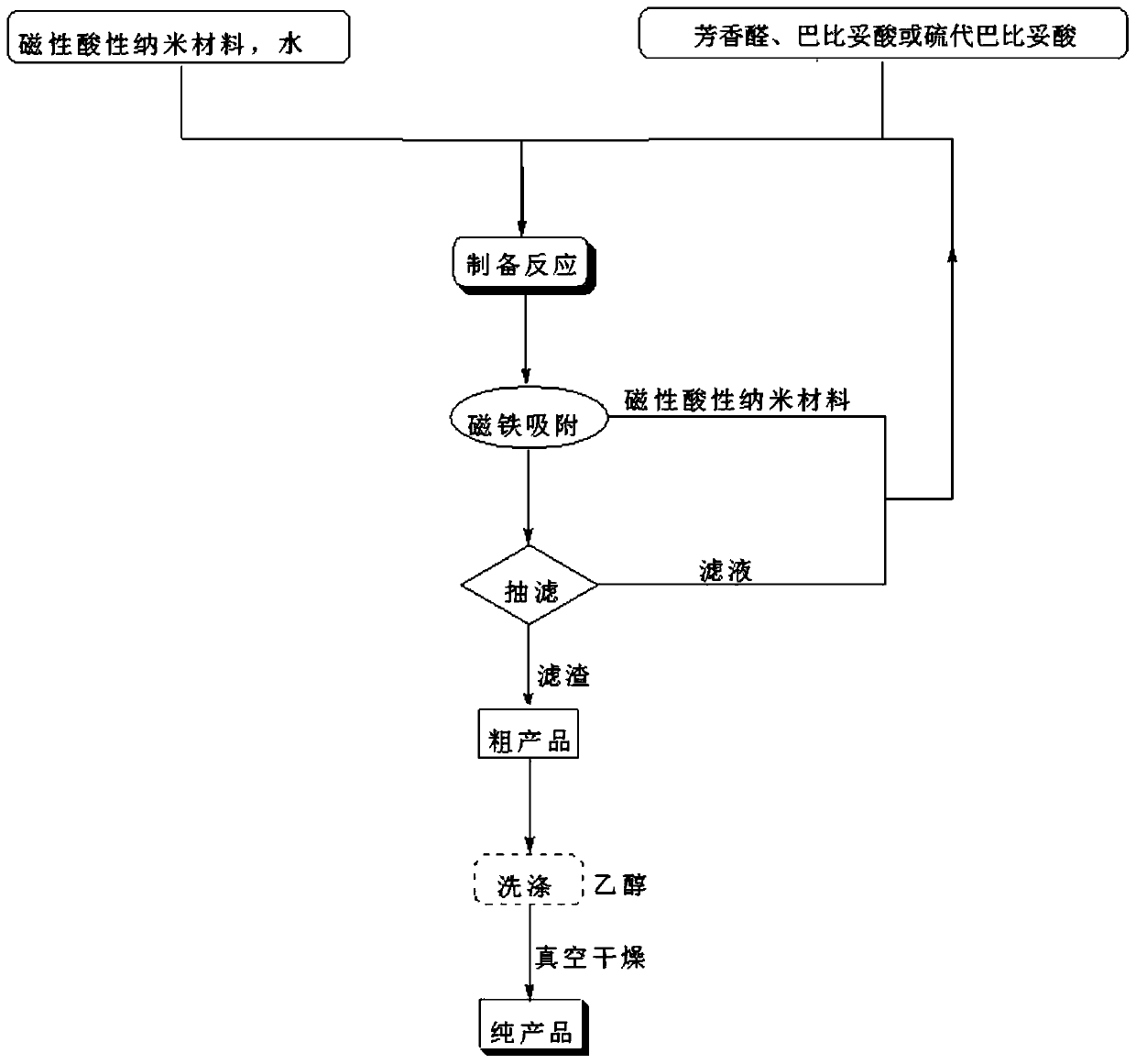
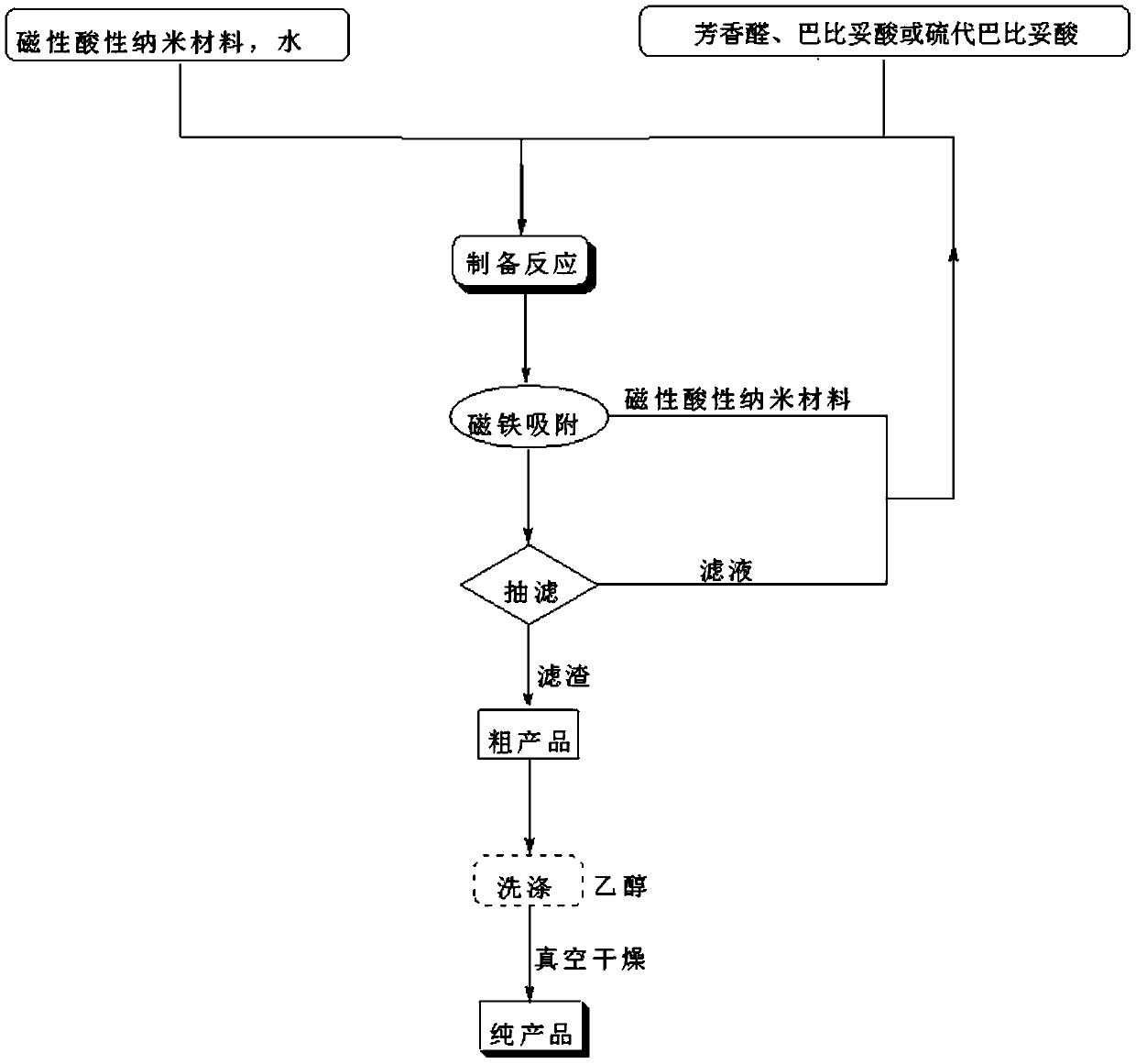
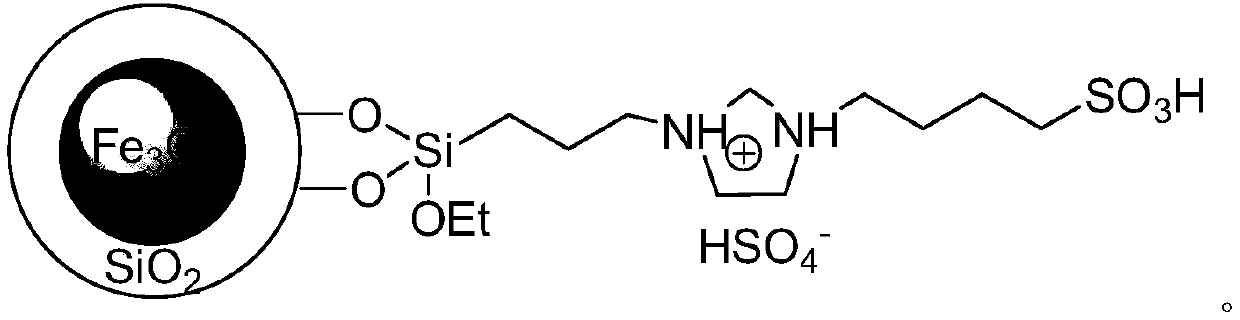
A kind of 5-arylene barbituric acid derivative, preparation method of the derivative and magnetic acidic nano material catalyst for preparation thereof
ActiveCN106622362BHigh catalytic activityReduce usageOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisFiltration
The invention discloses a 5-arylene barbituric acid derivative, a preparation method of the derivative and a magnetic acidic nanomaterial catalyst for its preparation, and belongs to the technical field of organic chemical synthesis. In the preparation reaction of the present invention, the molar ratio of aromatic aldehydes to barbituric acid or thiobarbituric acid is 1.0 to 1.4:1, and the molar amount of the magnetic acidic nanomaterial catalyst is 7 to 12% of the aromatic aldehyde used, in milliliters. The volume of reaction solvent water in millimoles is 4 to 7 times the molar amount of aromatic aldehyde in millimoles, the reaction temperature is 80 to 85°C, and the reaction time is 4 to 14 minutes. After the reaction is completed, use a magnet to absorb the catalyst while it is hot. The remaining reaction solution was cooled to room temperature and filtered with suction. The filter residue was washed with ethanol and dried under vacuum to obtain the 5-arylene barbituric acid derivative. The invention has the characteristics of low catalyst loss, high recycling times, simple and convenient operation of the entire preparation process, high degree of greenness, etc., and is convenient for large-scale industrial application.
Owner:东港智科产业园有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com