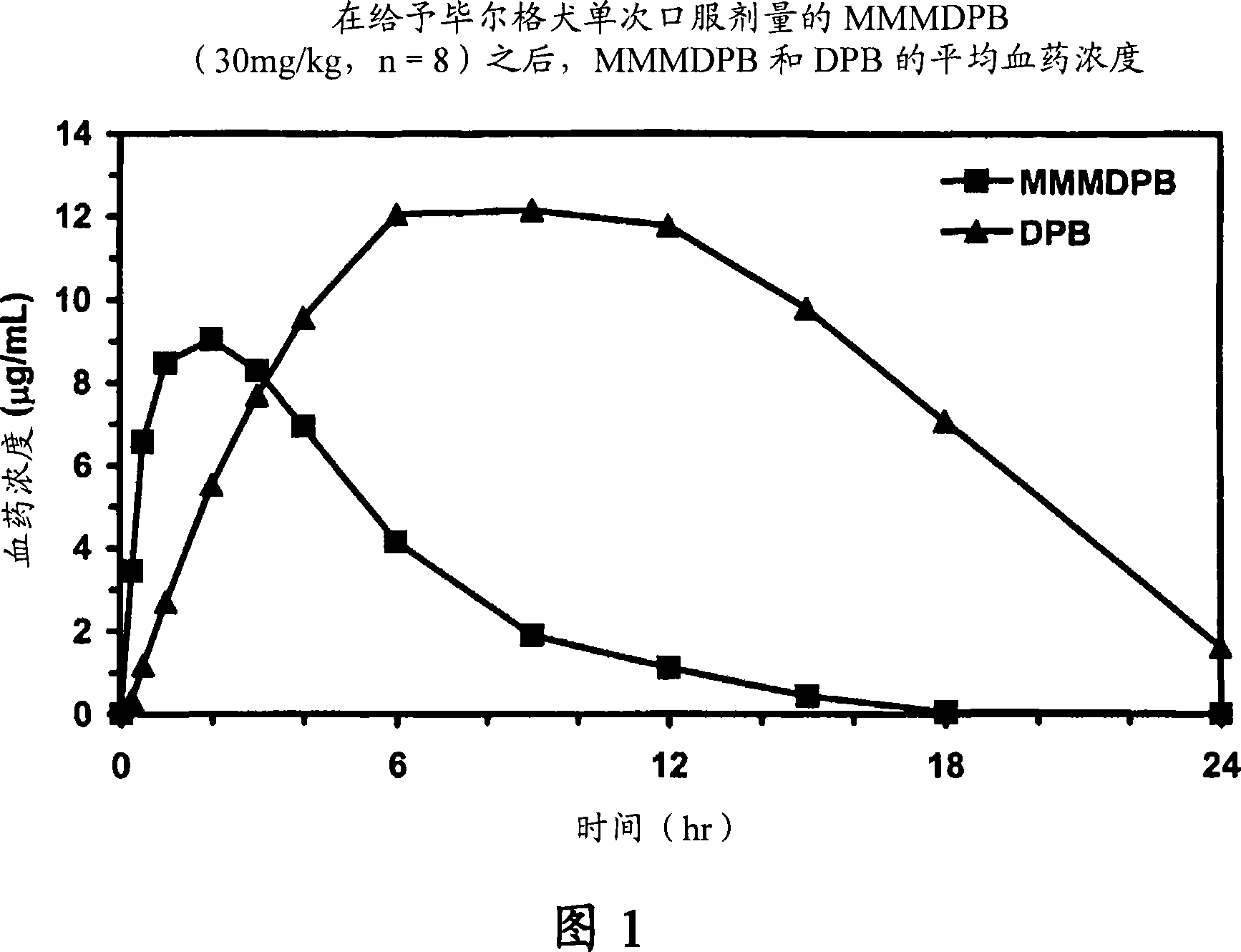
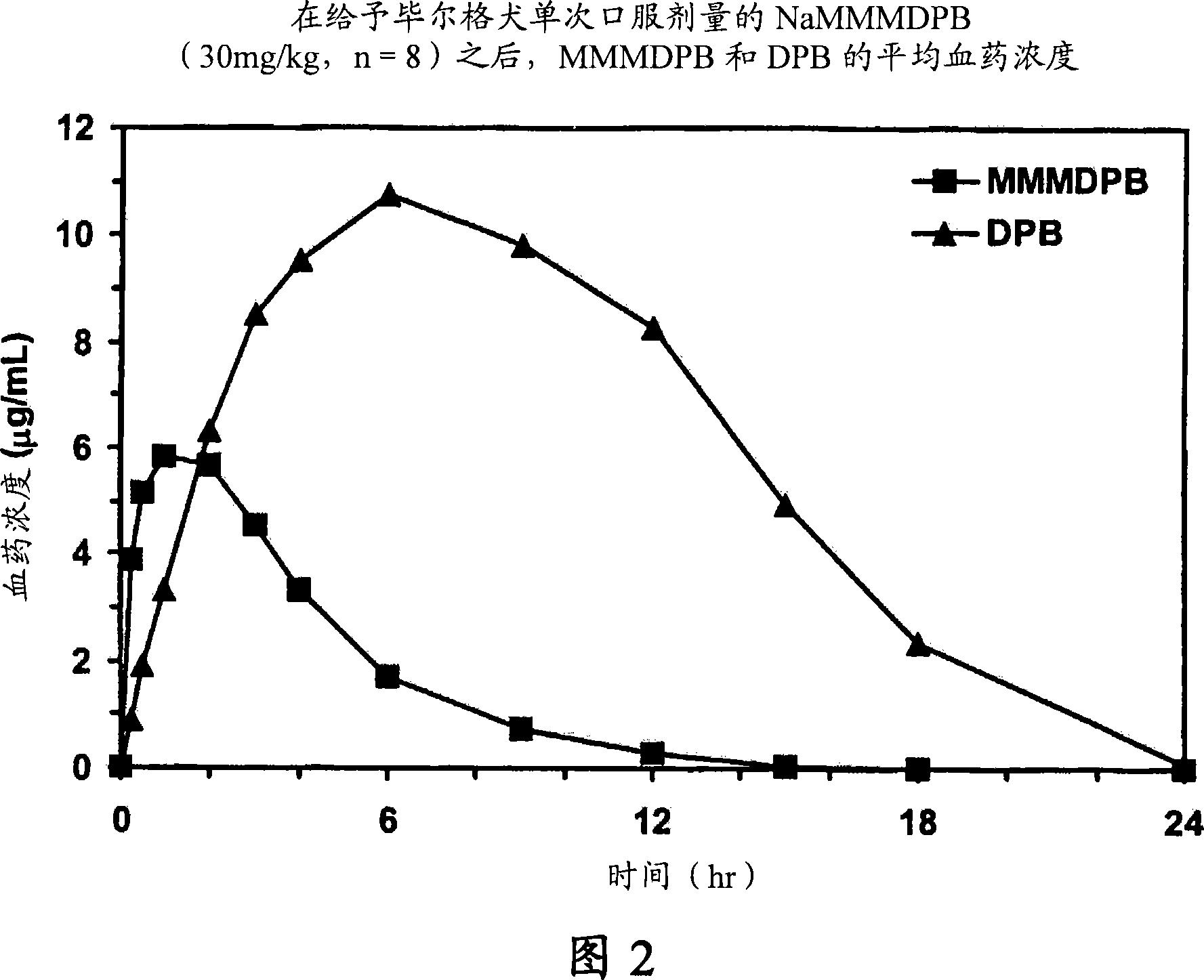
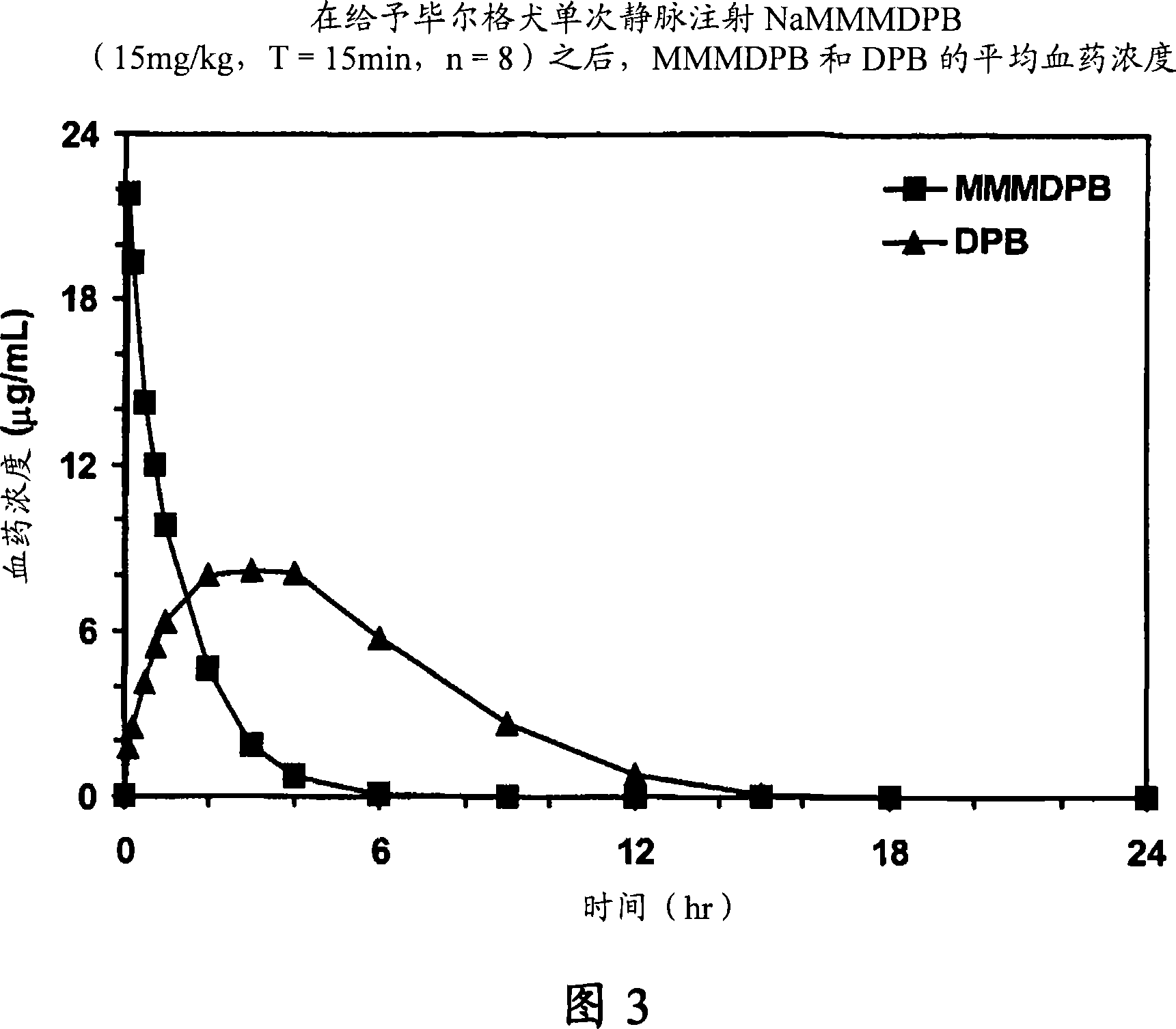
Composition and method for improving bioavailability and enhancing brain delivery of 5,5-diphenyl barbituric acid
A technology of diphenylbarbituric acid and sodium diphenylbarbiturate, which is applied in drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] The preparation of the DPB of embodiment 1 salt form
[0124] sodium salt
[0125] compound
molecular weight
Weight / grams)
Millimoles
volume
DPB
280
46.35
166
NaOH
40
6.60
165
THF
1,500ml
+150ml
DI water
25ml
[0126] THF: tetrahydrofuran; DI water: deionized water
[0127] operate:
[0128] DPB was dissolved in 1500ml THF. The cloudy solution was filtered through folded filter paper. A sodium hydroxide solution was prepared by dissolving in a mixture of 150 ml THF and 25 ml water. The sodium hydroxide solution was added dropwise to the DPB solution over 0.5 hours. DPB sodium salt formed and precipitated from solution. The mixture was stirred at room temperature for 2 hours, then cooled to 4°C and stirred at this temperature for a further 2 hours. The product was filtered and washed with cold THF. 42.57 g of wet product were obtained. The collec...
Embodiment 2
[0139] Other preparations of embodiment 2 DPB sodium salt
[0140] In Example 1, DPB was dissolved in THF, and then an equimolar amount of aqueous sodium hydroxide solution was added. This example uses a solution of sodium hydroxide in ethanol (ie, a 10% solution of NaOH in ethanol is prepared).
[0141] Operation (synthesized in THF)
[0142] DPB (7.0 g) was dissolved in 70 ml of THF at room temperature. A solution of 1 g NaOH (granules) in 10 ml absolute ethanol was added to the solution. The resulting mixture was stirred at room temperature. Cloudiness was observed immediately and precipitation of material was seen within minutes. The reaction mixture was then stirred for an additional 2 hours at room temperature. The product was filtered and washed with 15ml THF. The wet product was dried under vacuum at 105°C. Quantitative DPB was obtained. The purity of the product was 99.2%, and the water content measured by Karl Fisher method was 1.26%.
[0143] Operation (syn...
Embodiment 3
[0145] The preparation of the MMMDPB of embodiment 3 salt forms
[0146] sodium salt
[0147] 22 g MMMDPB (68 mmol) were suspended in 330 ml tert-butyl methyl ether and 10 ml methanol. The suspension was heated to 60°C and stirred at this temperature for 30 minutes. Then 13 ml of 30% sodium methoxide solution were added dropwise. During the addition, the suspension became a clear solution and after some time the product started to precipitate. After complete addition of the base, the reaction mixture was cooled to room temperature and stirred for an additional 4 hours. Filtration gave very fine crystals of the sodium salt.
[0148] The product (NaMMMDPB) was dried in a vacuum oven at 60°C for 3 hours. 18.4 g of dry sodium salt were obtained with a purity of 98.6% by HPLC analysis. Yield: 78.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com