Method for synthesizing 5-substituted barbituric acid derivative under catalysis of rare earth chloride
A rare earth chloride, barbituric acid technology, applied in the direction of organic chemistry, can solve the problems of many side reactions, harsh reaction conditions, low yield, etc., to achieve good substrate universality, simple synthesis method, and easy application Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
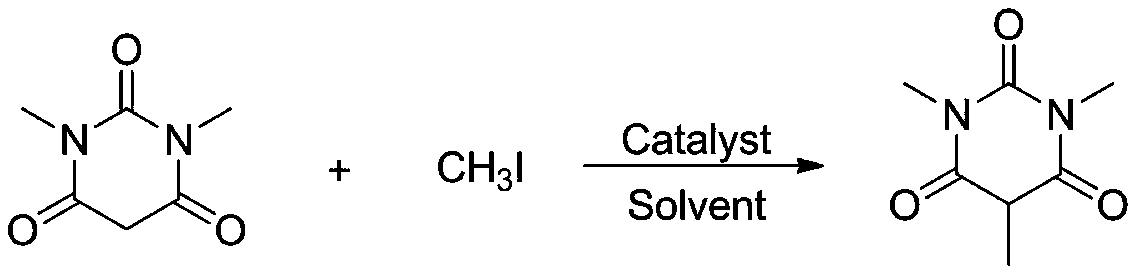
[0024]
[0025] Add 1.0mmol of 1,3-dimethylbarbituric acid, 1.0mmol of methyl iodide, and catalyst yttrium trichloride YCl to the reaction tube in sequence. 3 0.05 mmol, and 2 mL of solvent methanol was added, and the reaction was carried out at room temperature for 8 hours. After the reaction, the reaction solution was concentrated and separated by column chromatography to obtain the corresponding product with an isolation yield of 87%. 1 H NMR (400MHz, CDCl 3 ) δ: 4.78-4.72 (m, 1H), 3.40 (s, 6H), 1.05 (d, J=6.2Hz, 3H). HRMS theoretical value C 7 h 11 N 2 o 3 (M+H) + : 171.0770, actual measured value: 171.0775.
Embodiment 2
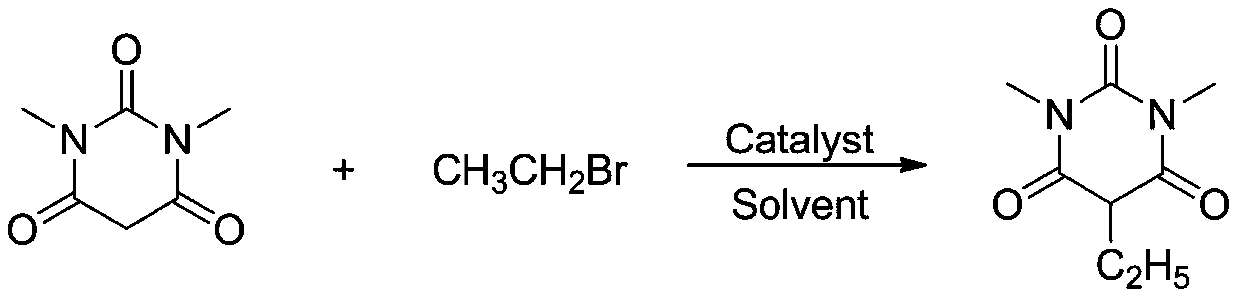
[0027]
[0028] Add 1.0mmol of 1,3-dimethylbarbituric acid, 1.2mmol of ethyl bromide, catalyst YbCl 3 0.02 mmol, and 2 mL of solvent methanol was added, and the reaction was carried out at room temperature for 6 hours. After the reaction, the reaction solution was concentrated and separated by column chromatography to obtain the corresponding product with an isolation yield of 85%. 1 H NMR (400MHz, CDCl 3 ) δ: 4.75(t, J=5.8Hz, 1H), 3.42(s, 6H), 1.09-1.05(m, 2H), 0.95(t, J=6.0Hz, 3H). HRMS theoretical value C 8 h 13 N 2 o 3 (M+H) + : 185.0926, actual measured value: 185.0930.
Embodiment 3
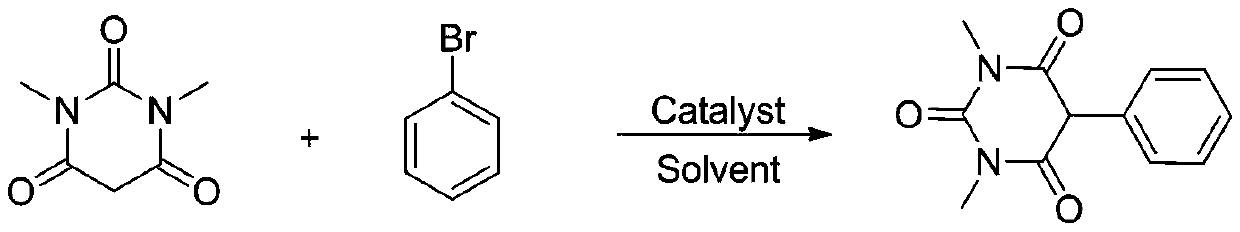
[0030]
[0031] Add 1.0mmol of 1,3-dimethylbarbituric acid, 1.2mmol of bromobenzene, catalyst ytterbium trichloride YbCl in the reaction tube 3 0.05 mmol, and 2 mL of solvent ethanol was added, and the reaction was carried out at room temperature for 10 hours. After the reaction, the reaction solution was concentrated and separated by column chromatography to obtain the corresponding product with an isolation yield of 90%. 1 H NMR (400MHz, CDCl 3 ) δ: 7.41-7.34 (m, 3H), 7.23 (dd, J=7.4, 1.8Hz, 2H), 4.67 (s, 1H), 3.36 (s, 6H). HRMS theoretical value C 12 h 13 N 2 o 3 (M+H) + : 233.0926, actual measured value: 233.0922.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com