Method for greenly synthesizing 5-hydroxy-5-alkyl disubstituted barbituric acid derivative by amine catalysis of air oxidation
A technology of dimethyl barbituric acid and air oxidation, applied in the direction of organic chemistry, etc., to achieve the effects of easy control and realization, mild reaction conditions and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of substrate
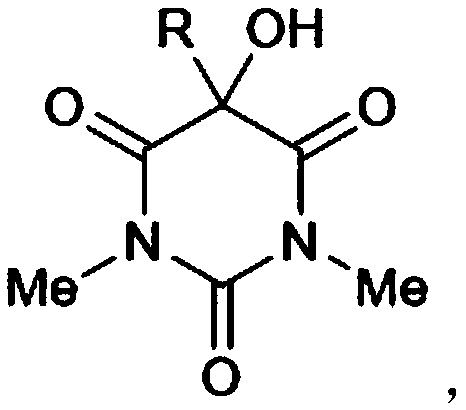
[0026] (1) Preparation of 5-aryl-1,3-dimethylbarbituric acid, the reaction equation is:
[0027]
[0028] Specific steps are as follows:
[0029] a. Add a stir bar, CuI (5.0 mol%), 2-picolinic acid (10.0 mol%), and Cs to a dry three-necked flask in sequence 2 CO 3 (3.0 mmol) and aryl iodide (solid) (1.0 mmol), then replaced with nitrogen three times to remove the air in the reaction flask. Add anhydrous 1,4-dioxane (2.0mmol) and diethyl malonate (0.3mL) to the reaction flask, then seal the reaction flask and place it in a preheated oil bath at 70℃. The reaction process was monitored by layer chromatography (TLC) and cooled to room temperature after the reaction. After the reaction, the reaction solution was separated and extracted with ethyl acetate (20 mL×3) and saturated ammonium chloride solution (10 mL). Anhydrous Na for organic layer 2 SO 4 (3g) Dry, filter, and concentrate. The crude product is subjected to rapid column separation...
Embodiment 2
[0036] Example 2 Oxidation reaction of 5-aryl-1,3-dimethylbarbituric acid derivatives
[0037] (1) 5-aryl-1,3-dimethylbarbituric acid oxidation, the reaction equation is:
[0038]
[0039] Specific steps are as follows:
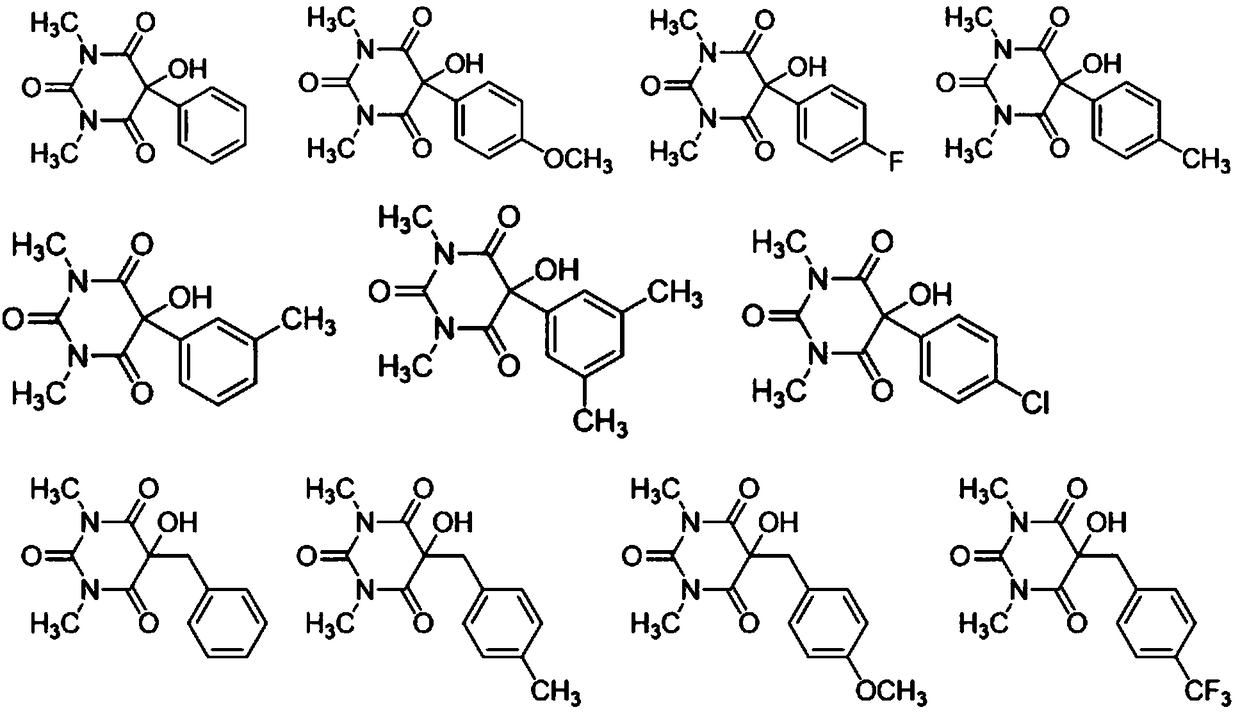
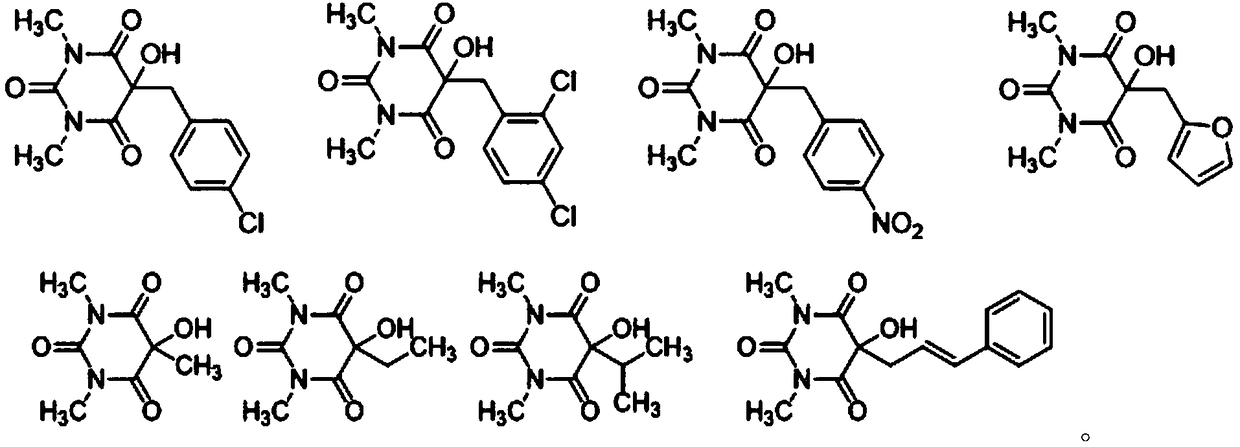
[0040] Combine hexamethyleneimine (HMTA) (2mol%), N,N-dimethylformamide (DMF) (0.3mL) and 5-aryl-1,3-dimethylbarbituric acid (11a -11g) (0.2mmol) was added to the reaction flask, and then stirred at room temperature (25°C), the reaction was monitored by TLC, and the reaction process is shown in reaction scheme 1. After the reaction, the target product (12a-12g) was obtained directly by silica gel column chromatography (petroleum ether / ethyl acetate=5 / 1).
[0041]
[0042] (2) 5-benzyl-1,3-dimethylbarbituric acid oxidation, the reaction equation is:
[0043]
[0044] Specific steps are as follows:
[0045] The N,N-diethylethylamine (Et 3 N) (10mol%), tetrahydrofuran (THF) (0.4mL) and 5-benzyl-1,3-dimethylbarbituric acid (13a-13h) (0.2mmol) were added to the reaction fl...
Embodiment 3
[0050] Example 3 Screening of oxidation reaction conditions for 5-aryl-1,3-dimethylbarbituric acid derivatives
[0051] Select 5-phenyl-1,3-dimethylbarbituric acid as the model substrate to screen the reaction conditions of α-CH hydroxylation. The reaction process is shown in Reaction Route 4, and the selection of reaction conditions is shown in Table 1 below. . Firstly, 20mol% DMAP was selected as the catalyst, and the solvent was initially explored. It was found that when DMF was the solvent, the reaction effect was the best. The reaction only required 12h to achieve the complete conversion of the raw materials and produce the target product 12a (97%) (Table 1 Record in 1). For other solvents, when the reaction time is extended to 24h, the complete conversion of the raw materials still cannot be achieved, resulting in a relatively low yield (records 2-9 in Table 1). When toluene or dichloromethane is selected as the solvent, the reaction time is also controlled at 24h, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com