A kind of 5-arylene barbituric acid derivative, preparation method of the derivative and magnetic acidic nano material catalyst for preparation thereof
A barbituric acid and nanomaterial technology, applied in the field of magnetic acid nanomaterial catalyst, can solve the problems of large loss of ionic liquid catalyst, complicated product purification process, low utilization rate of raw materials, etc. Purity, reduction of side reactions and impurities, and the effect of shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
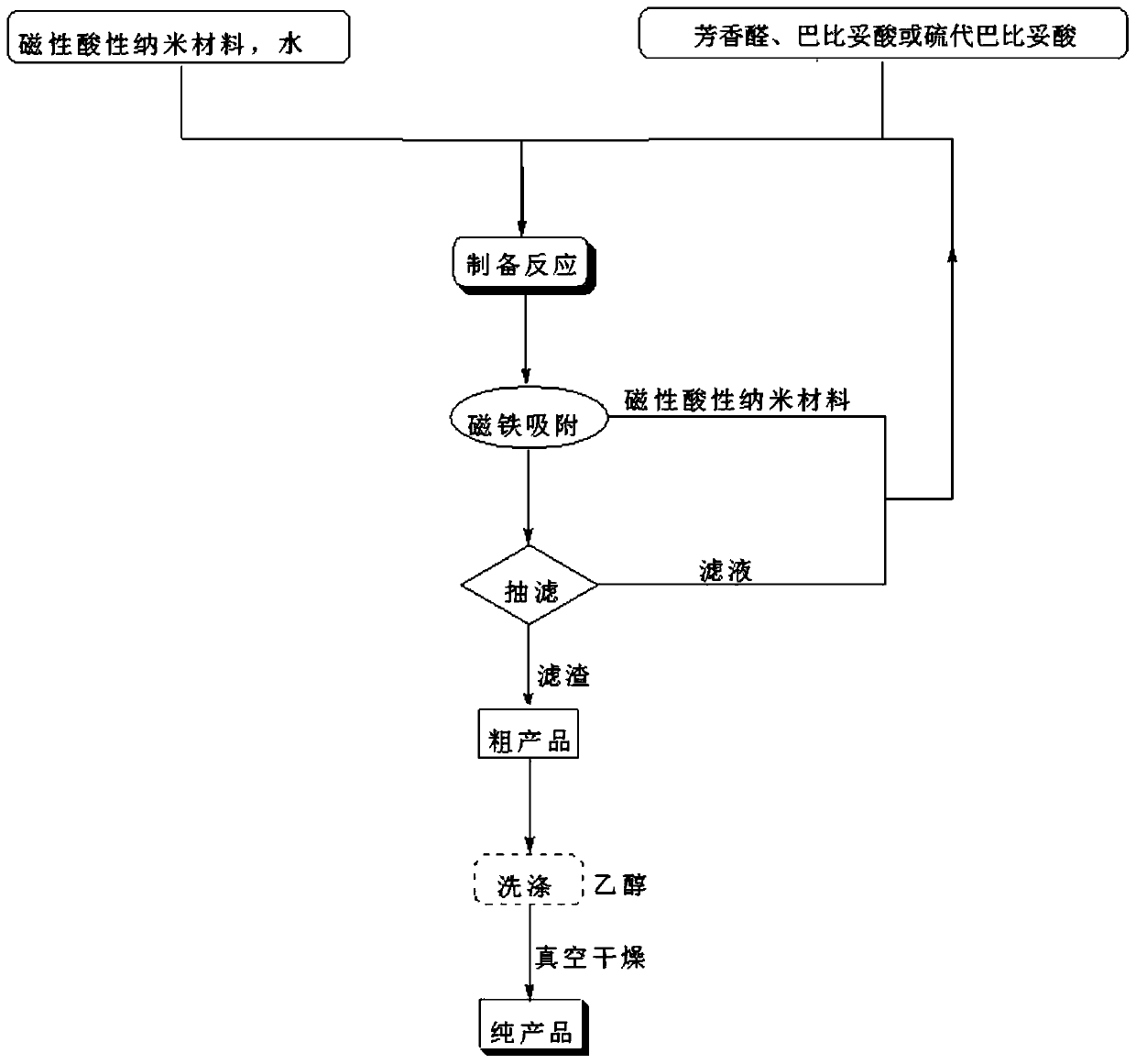
[0035] The technical process of the above-mentioned preparation method of 5-arylene barbituric acid derivative of the present invention is as follows figure 1 As shown, the specific steps are:
[0036] (1) Weigh the reaction raw materials aromatic aldehyde and barbituric acid or thiobarbituric acid according to the molar ratio of 1.0 to 1.4:1;
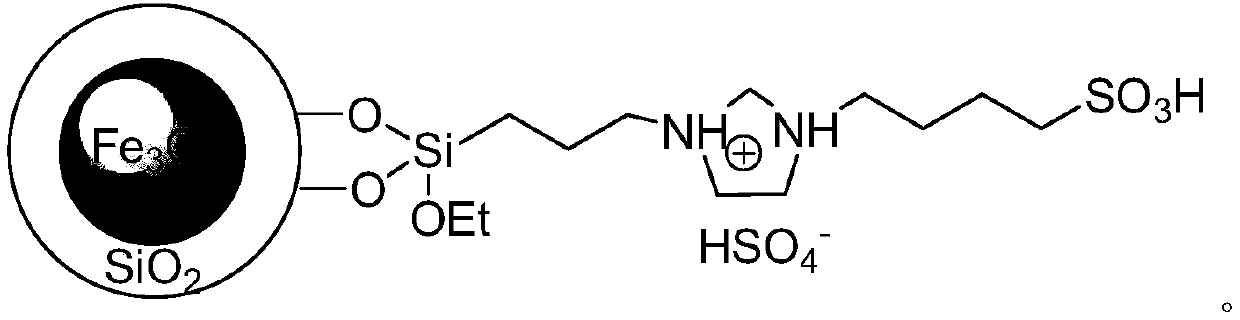
[0037] (2) Add the weighed aromatic aldehyde and barbituric acid or thiobarbituric acid into water respectively, mix them evenly and then continue to add magnetic acidic nanomaterials as catalysts, and heat to 80-85°C under magnetic stirring conditions. °C, react for 4 to 14 minutes, and the reaction pressure is one atmosphere. Wherein, the molar weight of the above-mentioned added magnetic acid nano material catalyst is 7-12% of the aromatic aldehyde molar weight used, and the volume of the reaction solvent water in milliliters is 4-7 times of the aromatic aldehyde molar weight in millimoles .
[0038] Because the type of catalyst ...
Embodiment 1
[0044] 1mmol of p-chlorobenzaldehyde, 1mmol of barbituric acid and 0.26g of magnetic acidic nanomaterials were added to 5ml of water in a 50ml single-necked round bottom flask with a stirring bar and a condenser. Heated to 80°C under magnetic stirring, reacted for 4 minutes, detected by TLC (thin plate chromatography), the raw material point disappeared, and the catalyst was adsorbed by a magnet while it was hot, and the remaining reaction solution was cooled to room temperature, crushed the precipitated solid, stood still, and suction filtered. The filter residue was washed with ethanol and vacuum-dried to obtain 5-(4-chlorobenzylidene)pyrimidine-2,4,6-trione with a yield of 91%, and the adsorbed catalyst was put into the filtrate to reconstitute the catalytic system , repeated use after directly adding p-chlorobenzaldehyde and barbituric acid.
[0045] The performance parameters of 5-(4-chlorobenzylidene)pyrimidine-2,4,6-trione obtained in the present invention are as follow...
Embodiment 2
[0047] 1.2mmol of p-chlorobenzaldehyde, 1mmol of thiobarbituric acid and 0.26g of magnetic acidic nanomaterials were respectively added to a 50ml single-necked round bottom flask containing 5ml of water with a stirring bar and a condenser tube. Heated to 82°C under magnetic stirring, reacted for 7 minutes, detected by TLC (thin plate chromatography), the raw material point disappeared, adsorbed the catalyst with a magnet while it was hot, cooled the remaining reaction solution to room temperature, crushed the precipitated solid, stood still, and suction filtered. The filter residue was washed with ethanol and vacuum-dried to obtain 5-(4-chlorobenzylidene)-2-sulfanyl-2H-pyrimidine-4,6-dione with a yield of 89%. The adsorbed catalyst was put into The catalytic system is reconstituted in the filtrate, and p-chlorobenzaldehyde and thiobarbituric acid are directly added for repeated use.
[0048] The performance parameters of 5-(4-chlorobenzylidene)-2-thio-2H-pyrimidine-4,6-dione o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com