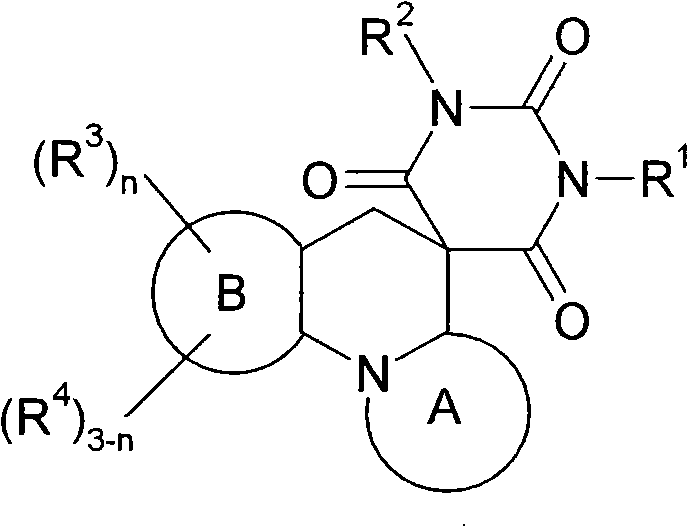
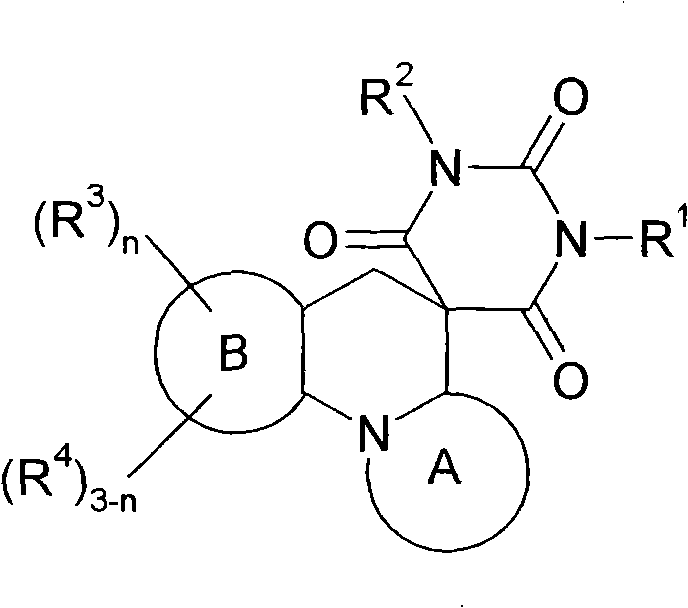
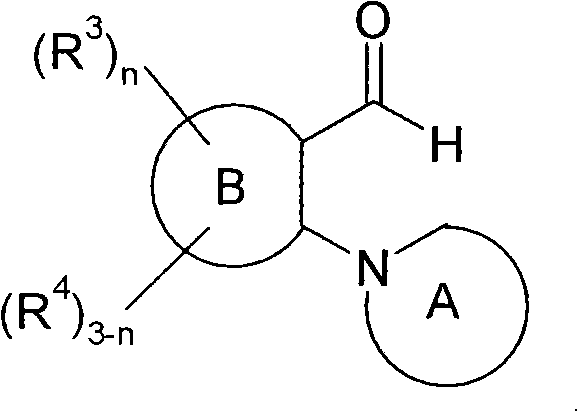
Spiro condensed barbituric acid derivatives for use as antibacterial
A compound and pharmaceutical technology, applied in the field of substituted heterocycles, can solve problems such as weak antibacterial agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1229] (6aS, 7S, 9R)-rel-7,9-dimethyl-3-(1,3,4-thiadiazol-2-yl)-6a,7,9,10-tetrahydro -2'H, 5H-spiro[1,4-oxazino[4,3-a][1,8]naphthyridine-6,5'-pyrimidine]-2',4',6'(1' H,3′H)-trione
[1230] 2-[(2R,6S)-2,6-dimethylmorpholin-4-yl]-5-(1,3,4-thiadiazol-2-yl)pyridine-3-carbaldehyde (intermediate 114, 32 mg, 0.0107 mmol) and barbituric acid (16.4 mg, 0.0128 mmol) in anhydrous IPA (2 mL) were heated to 90° C. for 12 hours. The reaction mixture was cooled to room temperature and concentrated. The residue thus obtained was purified on a neutral aluminum column using a gradient of ethyl acetate / petroleum ether to give the product as a solid. MS(ES)MH + : 415.0, for C 18 h 18 N 6 o 4 S; 1 H NMR (400MHz, DMSO): δ1.0(d, 3H), 1.2(d, 3H), 2.8(m, 1H), 2.9(d, 1H), 3.6(m, 3H), 4.0(d, 1H ), 5.1 (dd, 1H), 7.8 (s, 1H), 8.6 (d, 1H), 9.5 (s, 1H), 11.7 (s, broad peak, 2H).
Embodiment 2
[1233] 3-Bromo-7,9-dimethyl-5,6a,7,8,9,10-hexahydro-1'H-spiro[pyrido[1,2-a][1,7]naphthyridine -6,5'-pyrimidine]-2',4',6'(3'H)-trione
[1234] Starting material: Intermediate 19.
[1235] MS(ES)MH + :407 for C 17 h 19 BrN 4 o 3 .
[1236] 1 H NMR (300 MHz, DMSO): 0.7 (2d, 3H), 0.9 (2d, 3H, 4:1 ratio), 1.0 (q, 1H), 1.4-1.8 (m, 3H), 2.7 (m, 1H), 2.8 and 2.9 (2d, 1H, 4:1 ratio), 3.2 (m, 1H), 3.4 (m, 1H), 3.6 (m, 1H), 3.9-4.1 (m, 1H), 7.1 (2s, 1H, 4:1 ratio), 7.9 (2s, 1H, 4:1 ratio), 11.5 (2s, 1H, 4:1 ratio), 11.7 (2s, 1H, 4:1 ratio).
Embodiment 3
[1238] (6aS,7S,9R)-rel-3-bromo-7,9-dimethyl-6a,7,9,10-tetrahydro-1′H,5H-spiro[[1,4]oxazine And[4,3-a][1,7]naphthyridine-6,5'-pyrimidine]-2',4',6'(3'H)-trione
[1239] Starting material: Intermediate 18.
[1240] MS(ES)MH + :409 for C 16 h 17 BrN 4 o 4 .
[1241] 1 H NMR (300MHz, DMSO): 0.95(d, 3H), 1.1(d, 3H), 2.7-3.0(m, 2H), 3.4-3.7(m, 4H), 4.1(d, 1H), 7.1(s , 1H), 8.0(s, 1H), 11.6(s, 1H), 11.8(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com