Barbituric acid derivative, preparation method thereof, and application of derivative in data encryption and decryption
A barbituric acid and data encryption technology, applied in data encryption and decryption, in the field of barbituric acid derivatives and preparation, can solve problems such as limiting practical applications, and achieve excellent fluorescence activity and enhanced crystallization-induced emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of compound (referred to as CB-1) shown in formula II:
[0056] 9-phenylcarbazole-3-carbaldehyde and 1,3-dimethylbarbituric acid (molar ratio of 1:1) were added to ethanol (10mL), heated to reflux for 3 hours, after the reaction was completed, the reaction After the end, the material was rotary evaporated to obtain a solid, which was purified by silica gel column chromatography (ethyl acetate:petroleum ether 2:1) to obtain the product, which was designated as CB-1, and the yield was 90%.
[0057] 1 H NMR (400MHz, Chloroform-d) δ9.34(d, J=1.7Hz, 1H), 8.81(s, 1H), 8.40(d, J=8.9, 1.8Hz, 1H), 8.26(d, J= 7.7Hz, 1H), 7.66(t, J=7.7Hz, 2H), 7.60–7.53(m, 3H), 7.51–7.46(m, 1H), 7.41(t, J=5.4Hz, 3H), 3.46( d, J = 1.5 Hz, 6H) (Fig. S1). FT-IR (KBr, cm-1): 1722 (C = O).
Embodiment 2
[0059] Synthesis of compound (referred to as CB-2) shown in formula I:
[0060] 9-phenylcarbazole-3-carbaldehyde and 1,3-diphenylbarbituric acid (1:1 molar ratio) were added to ethanol (10mL), heated to reflux for 3 hours, after the reaction was completed, the reaction After the end, the material was rotary evaporated to obtain a solid, and the solid was purified by silica gel column chromatography (ethyl acetate:petroleum ether 2:1) to obtain the product, which was designated as CB-2, and the yield was 90%.
[0061] 1 H NMR (400MHz, Chloroform-d) δ9.32(s,1H),8.95(s,1H),8.49(d,J=9.6Hz,1H),8.23(d,J=7.7Hz,1H),7.69 –7.61(m,3H),7.53(d,J=11.4Hz,6H),7.47(t,J=7.6Hz,4H),7.37(d,J=8.0Hz,6H)(Fig.S3).FT -IR(KBr,cm -1 ): 1737 (C=O).
[0062] The synthetic routes of CB-1 and CB-2 are as follows:
[0063]
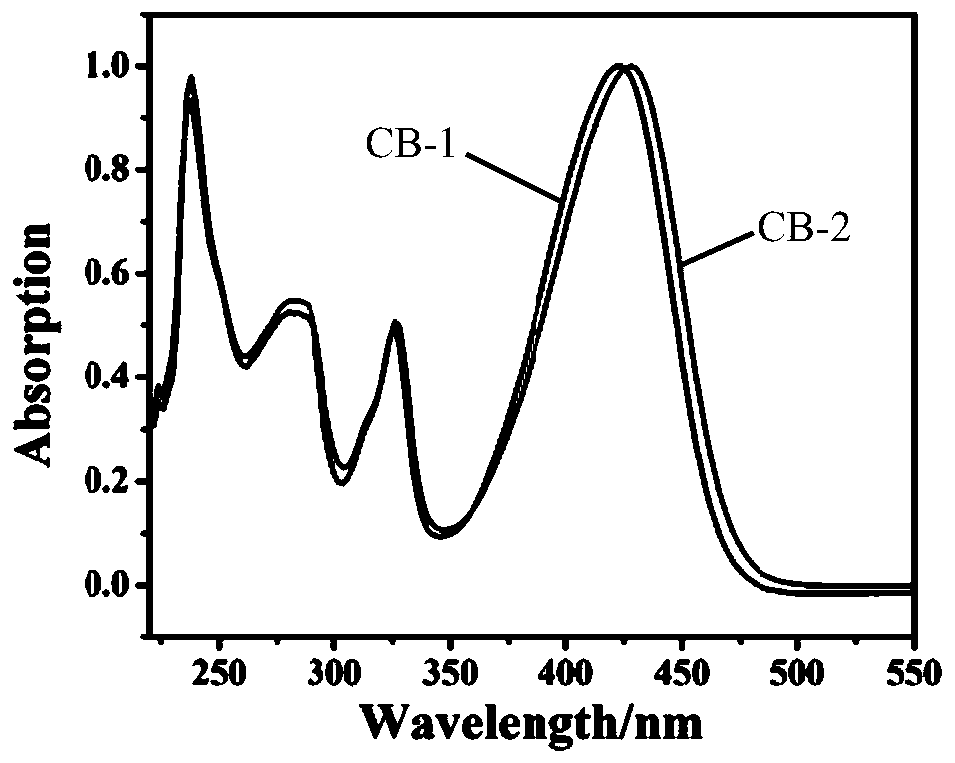
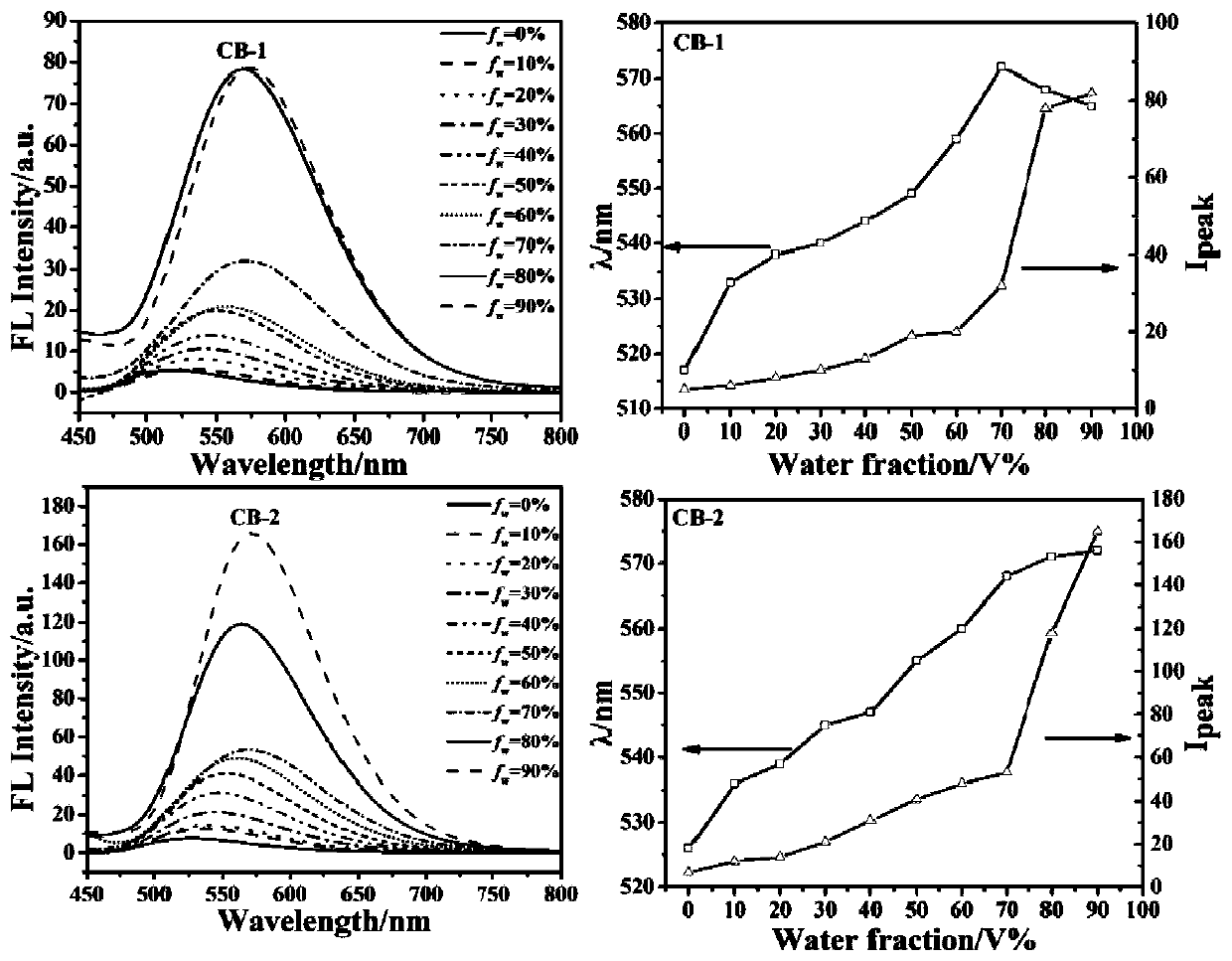
[0064] CB-1, CB-2 prepared by embodiment 1, 2 are characterized as follows:
[0065] The ultraviolet-visible absorption spectrum of CB-1, CB-2 in tetrahydrofuran (THF) solution i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com