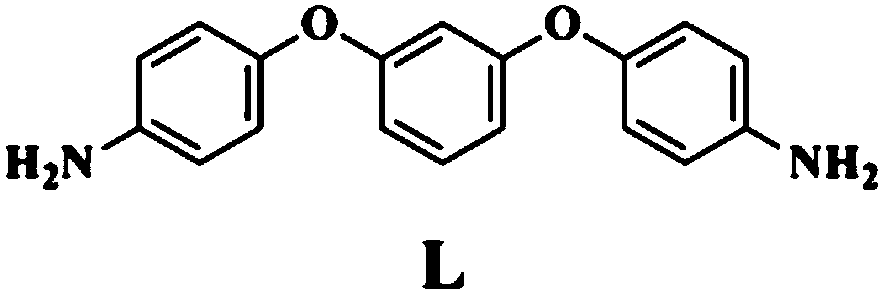
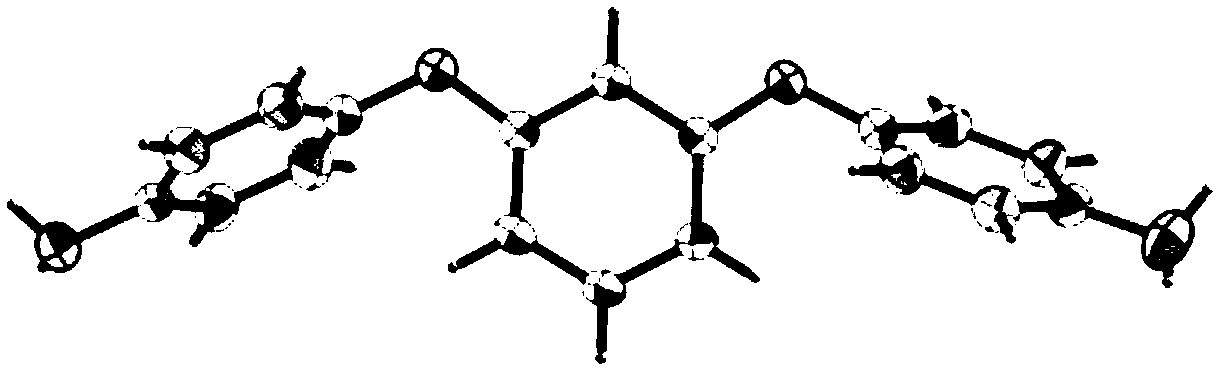
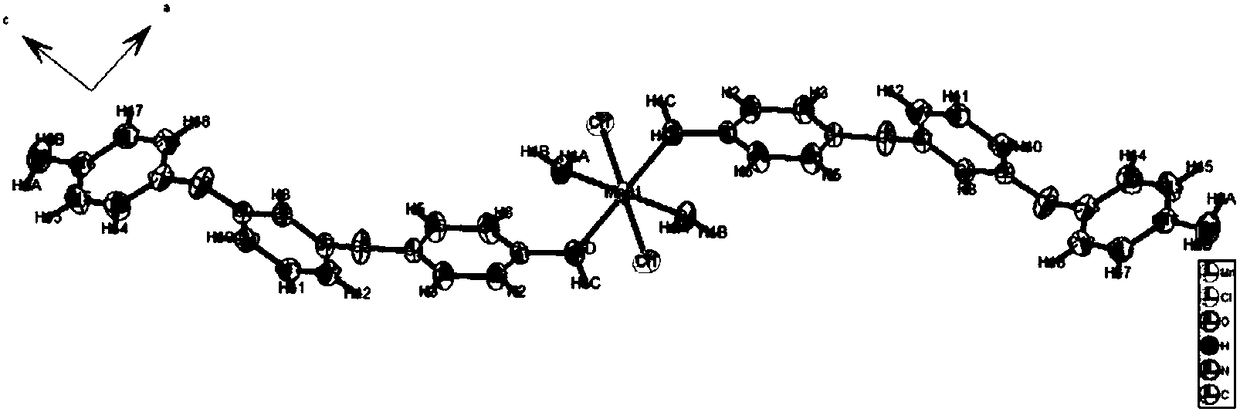
MnII-based belt-shaped crossed compound as well as preparation method and application thereof
A cross-fitting and ribbon-shaped technology, which is applied in the field of ribbon-shaped cross-complex materials, can solve the problems of poor detection effect and high cost, and achieve the effects of simple preparation method, good fluorescence activity, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 is based on Mn Ⅱ ribbon cross complex
[0023] (1) The preparation method is as follows:
[0024] 1. Mix 0.0060g (0.03mmol) of MnCl 2 4H 2 O and 0.0176g (0.06mmol) of organic aromatic diamine ligand 1,3-bis(4-aminophenoxy)benzene (L) were put into 25mL Erlenmeyer flask respectively, and MnCl 2 Add 5mL of absolute ethanol and 3mL of dichloromethane into 1,3-bis(4-aminophenoxy)benzene, heat and stir to dissolve them completely. MnCl 2 Pour the absolute ethanol solution of 1,3-bis(4-aminophenoxy)benzene into the dichloromethane solution, and add 3 drops of distilled water to shake and mix well; seal the Erlenmeyer flask and leave it at room temperature In 10 days, reddish-brown powdery crystals were obtained, and then the powdery crystals were washed, filtered and dried to obtain Ⅱ The crude product of the ribbon cross-complex, the yield was 88%.
[0025] 2, will get based on Mn Ⅱ 0.03g of the crude product of the ribbon cross-complex was dissolved in ...
Embodiment 2
[0028] Embodiment 2 utilizes based on Mn Ⅱ Fluorescence Activity of Ribboned Cross Complexes for Detection of Aromatic Nitro Compounds
[0029] Method: the Mn-based Mn prepared in Example 1 Ⅱ Ribbon cross-complex grinding. In 10mL of acetonitrile, add 0.0075mg based on Mn Ⅱ The band-shaped cross-complexes can be dispersed evenly by ultrasonic to form a suspension.
[0030] Take 13 suspensions and put them into 13 groups of fluorescence cuvettes respectively, then add organic solvents dichloromethane (DCM), 1,4-dioxane (Diox) to these 13 groups of fluorescence cuvettes respectively , diethyl malonate (DM), ethyl acetate (EA), ethanol (EtOH), cyclohexane (He), methanol (MeOH), petroleum ether (PE), benzene (PhH), sec-butanol ( 2BA), nitrobenzene (NB), 2-nitrotoluene (2NT) and 3-nitrotoluene (3NT), stirred evenly, and detected by fluorescence spectrometer. Under the condition that the excitation light wavelength is 300nm, the emission light wavelength is 366nm for detection....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com