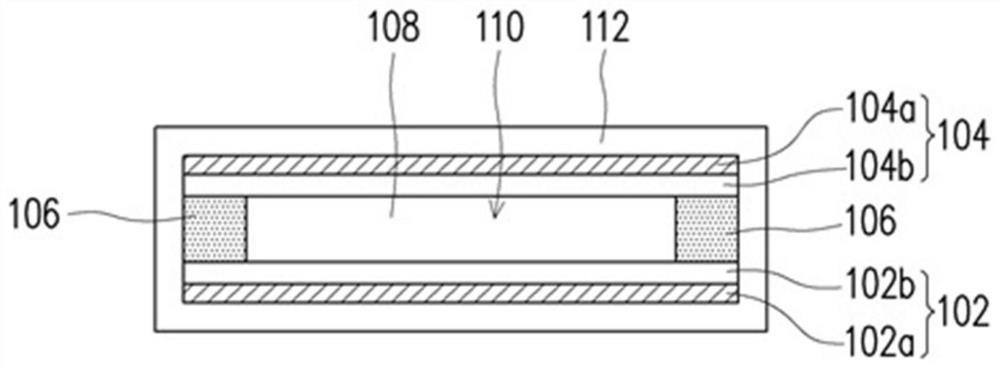
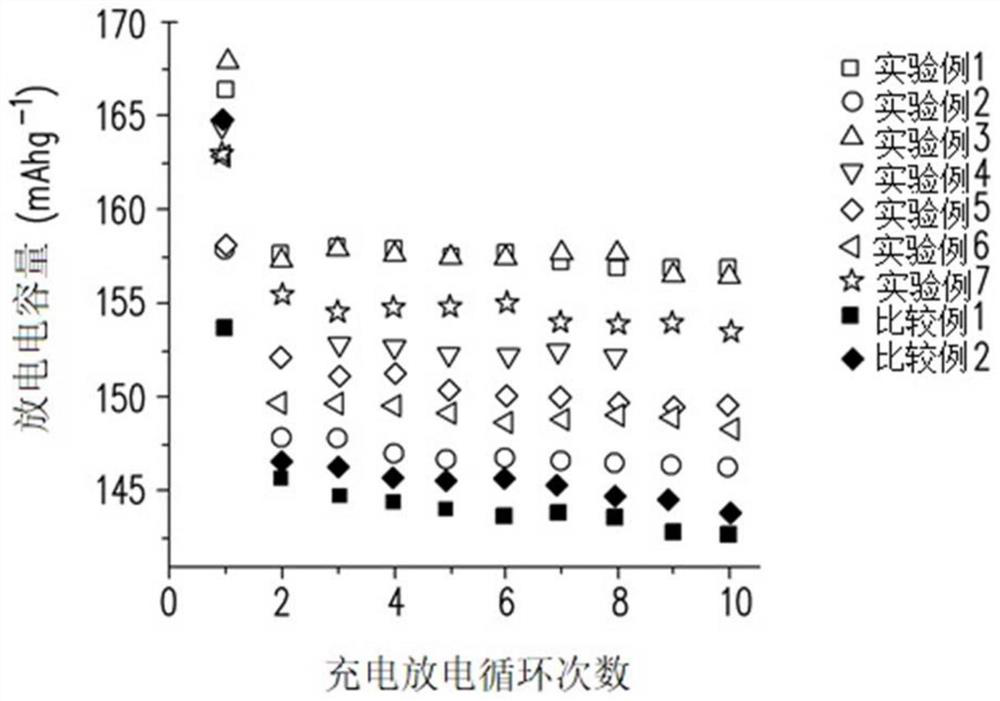
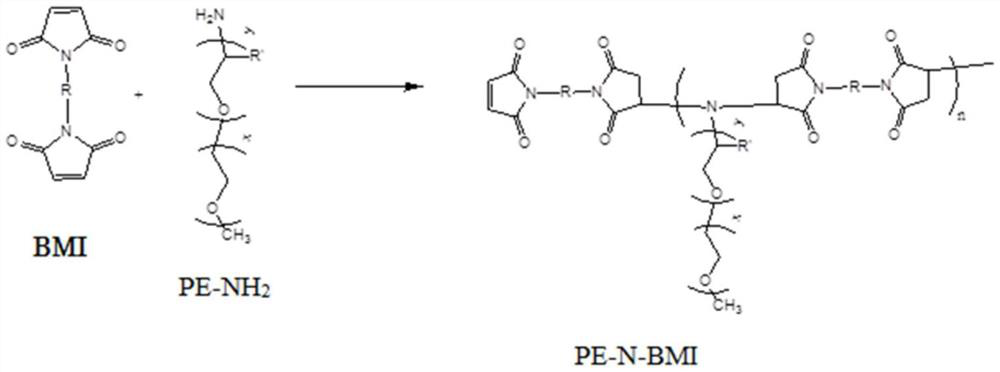
Polymer and lithium battery
A technology of polymers and compounds, applied in lithium batteries, battery electrodes, active material electrodes, etc., can solve problems such as cathode damage and achieve good capacitance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0047] Barbituric acid (BTA) and thiobarbituric acid (TBTA) were used as nucleophilic compounds, respectively. Barbituric acid (or thiobarbituric acid) and the above-mentioned prepolymer (PE-N-BMI) can undergo Myco addition reaction and free radical copolymerization reaction to form the polymer of the present invention.
[0048] Free Radical Polymerization:
[0049]
[0050] (X is O or S, n is the degree of polymerization)
[0051] Myco addition reaction:
[0052]
[0053] (X is O or S)
example 2
[0055] Cyanuric acid (CA) and thiocyanuric acid (TCA) were used as nucleophilic compounds, respectively. Cyanuric acid (or thiocyanic acid) and the above-mentioned prepolymer (PE-N-BMI) can undergo Myco addition reaction to form the polymer of the present invention.
[0056] Myco addition reaction:
[0057]
[0058] (X is O or S)
example 3
[0060] Monomaleimide (MI) was used as the nucleophilic compound. Monomaleimide and the above-mentioned prepolymer (PE-N-BMI) can undergo Michael addition reaction and free radical copolymerization reaction to form the polymer of the present invention.
[0061] Myco addition reaction:
[0062]
[0063] (n is the degree of polymerization)
[0064] Free Radical Polymerization:
[0065]
[0066] (n is the degree of polymerization)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com