Barbituric acid derivatives and preparation method thereof
A technology of barbituric acid and dimethyl barbituric acid, which is applied in the field of barbituric acid derivatives containing thiophene and fluorine substitution and their preparation, and achieves the effects of easy availability of raw materials, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
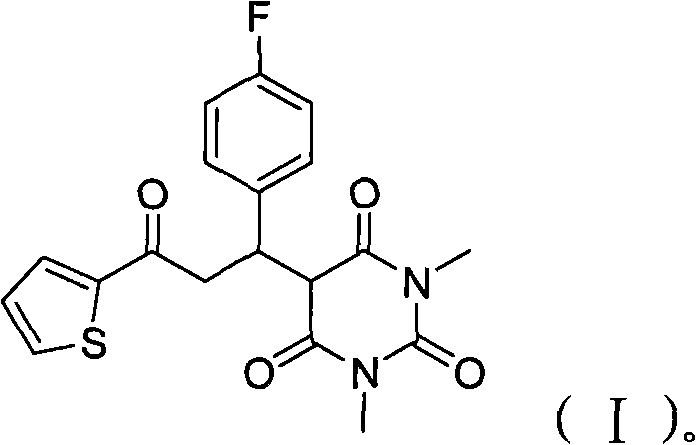
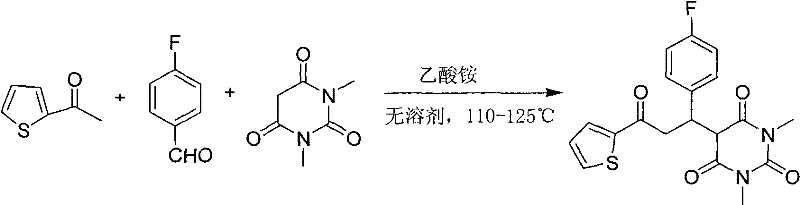
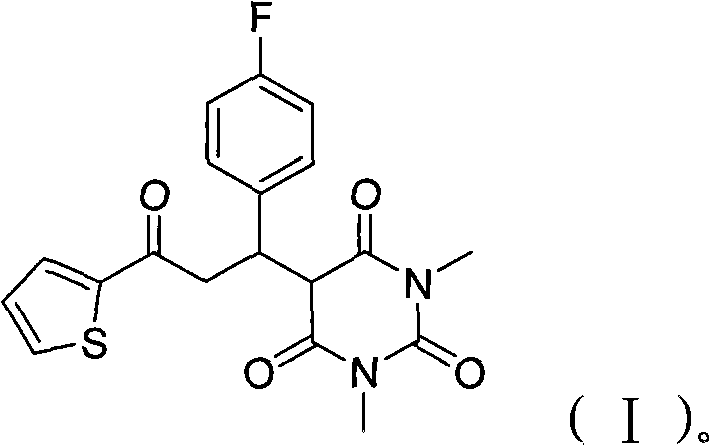
[0023] Put 1.26g of 2-acetylthiophene, 1.36g of p-fluorobenzaldehyde, 1.56g of 1,3-dimethylbarbituric acid and 0.1g of ammonium acetate in a 50ml reaction flask and mix evenly, heat and stir, and control the temperature At 110°C, reacted for 3 hours; after the reaction was completed, the reaction mixture was dissolved with 20 ml of dichloromethane, washed twice with 2 ml of water, dried over anhydrous sodium sulfate, then evaporated to remove the solvent, and recrystallized with 95% ethanol to obtain 3.30 g white solid 5-(1-(4-fluorophenyl)-3-oxo-3-(thiophen-2-yl)propyl)-1,3-dimethylbarbituric acid, yield 85 %.
[0024] After testing, the molecular formula of the product: C 19 h 17 FN 2 o 4 S, molecular weight: 388.41, appearance: white solid, melting point: 124-125°C.
[0025] 1 H NMR (CDCl 3 , 400MHz) δ: 7.83 (dd, 1H, J=0.8, 4.0Hz, ArH), 7.65 (dd, 1H, J=0.8, 5.2Hz, ArH), 7.15-7.08 (m, 3H, ArH), 6.98- 6.92(m, 2H, ArH), 4.38-4.34(m, 1H, CH), 4.02-3.93(m, 2H, CH), 3.49-...
Embodiment 2
[0029] 12.6g 2-acetylthiophene, 13.6g p-fluorobenzaldehyde, 15.6g 1,3-dimethylbarbituric acid and 1g ammonium acetate were placed in a 250 ml reaction flask and mixed uniformly, heated and stirred, and the temperature was controlled at 120°C, reacted for 2.5 hours; after the reaction was completed, the reaction mixture was dissolved with 200 ml of dichloromethane, washed twice with 200 ml of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and recrystallized with 95% ethanol to obtain the corresponding white Product 5-(1-(4-fluorophenyl)-3-oxo-3-(thiophen-2-yl)propyl)-1,3-dimethylbarbituric acid 33.6g, the yield is 87 %, melting point: 124-125°C.
Embodiment 3
[0031] 100.8g 2-acetylthiophene, 161.2g p-fluorobenzaldehyde, 124.8g 1,3-dimethylbarbituric acid and 10g ammonium acetate were placed in a 1000 ml reaction flask and mixed evenly, heated and stirred, and the temperature was controlled at 125°C, reacted for 2.5 hours; after the reaction was completed, the reaction mixture was dissolved with 2000 ml of dichloromethane, washed twice with 2000 ml of water, dried over anhydrous sodium sulfate, evaporated to remove the solvent, and recrystallized with 95% ethanol to obtain 328 g of a white solid 5-(1-(4-Fluorophenyl)-3-oxo-3-(thien-2-yl)propyl)-1,3-dimethylbarbituric acid, 84% yield, m.p. : 124-125°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com