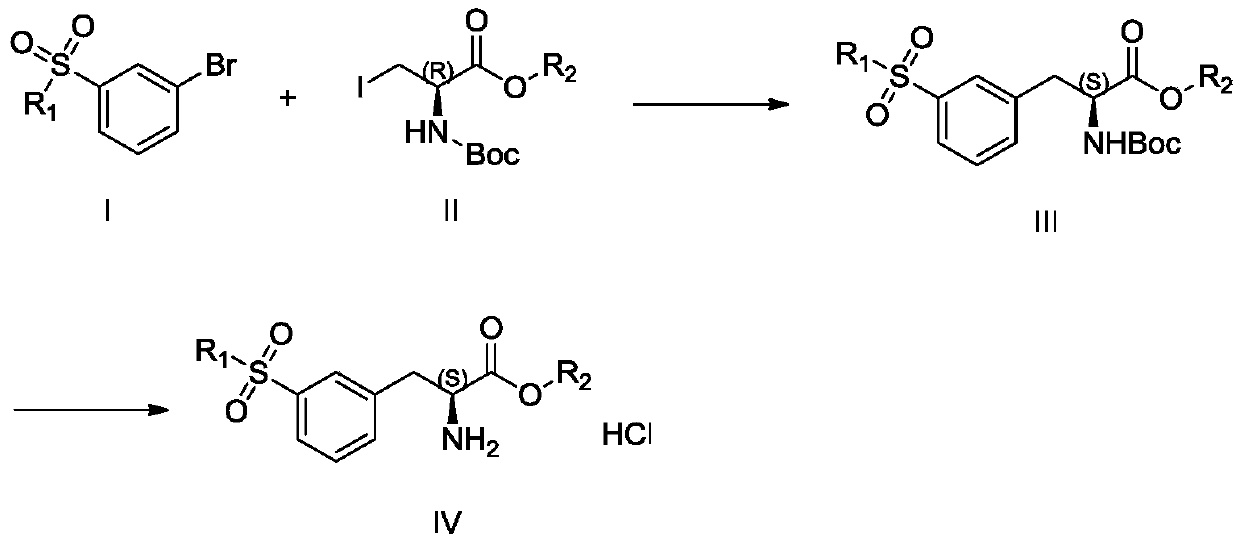
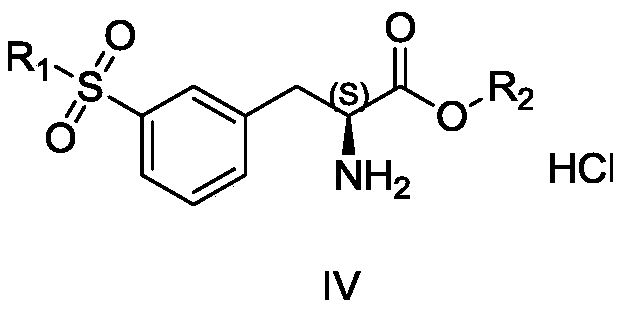
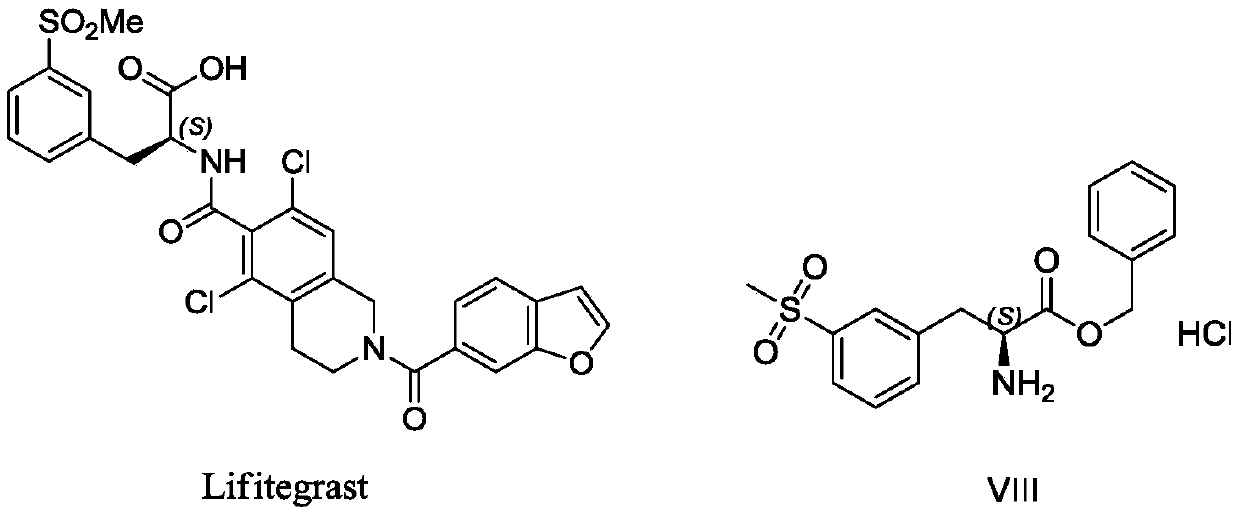
Preparation method of lifitegrast intermediate
A phosphorus ligand and compound technology, applied in the field of organic synthesis, can solve the problems of L-3-bromophenylalanine being expensive, unsuitable for industrial production, and long method routes, achieving low cost, suitable for industrial production, and reducing The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 20mL DMAC and 5.0g zinc powder into 100mL reaction bottle 1, replace with nitrogen three times, add dropwise 0.4g methanesulfonic acid and stir to raise the temperature to 60-70°C, keep it warm for 20-30min, then lower the temperature by 10-20°C, slowly add N- Boc-3-iodo-L-alanine benzyl ester (24.0 g) was dissolved in DMAC (20 mL) for about 4 hours, and the temperature was controlled at 20°C. After the dropwise addition, the reaction was stirred for 0.5-1 h, and the reaction was basically complete as detected by TLC or GC, and the obtained organozinc reagent was ready for use.
[0029] Add 20mL DMAC to 100mL reaction vial 2, add 10g 3-bromophenylmethyl sulfone, 389mg Pd 2 (dba) 3 , 259mg of tris(o-methylphenyl)phosphorus, replaced by nitrogen three times, then heated to 70-80°C, added dropwise the organozinc reagent in reaction bottle 1, after the addition was completed, kept stirring for 1 hour and sent to LC-MS. After the reaction was complete, the reaction sol...
Embodiment 2
[0031] Add 20mL DMAC and 5.0g zinc powder into 100mL reaction bottle 1, replace with nitrogen three times, add dropwise 0.4g methanesulfonic acid and stir to raise the temperature to 60-70°C, keep it warm for 20-30min, then lower the temperature by 10-20°C, slowly add N- Boc-3-iodo-L-alanine benzyl ester (24.0 g) was dissolved in DMAC (20 mL) for about 4 hours, and the temperature was controlled at 20°C. After the dropwise addition, the reaction was stirred for 0.5-1 h, and the reaction was basically complete as detected by TLC or GC, and the obtained organozinc reagent was ready for use.
[0032] Add 20mL DMAC to 100mL reaction vial 2, add 10g 3-bromophenylmethyl sulfone, 135mg Pd(OAc) 2 , 259mg of tris(o-methylphenyl)phosphorus, replaced by nitrogen three times, then heated to 70-80°C, added dropwise the organozinc reagent in reaction bottle 1, after the addition was completed, kept stirring for 1 hour and sent to LC-MS. After the reaction was complete, the reaction solutio...
Embodiment 3
[0034]Add 20mL DMAC and 5g zinc powder to the reaction bottle 1, replace with nitrogen three times, then add 0.4g methanesulfonic acid dropwise and stir to raise the temperature to 60-70°C, keep it warm for 20-30min, then lower the temperature by 10-20°C, slowly add N-Boc- A DMAC solution (20 mL) of 3-iodo-L-alanine methyl ester (19.5 g) was used for about 4 h at a temperature of 20° C. After the dropwise addition was completed, the reaction was stirred for 0.5-1 h. TLC or GC detects that the basic reaction is complete, and the obtained organozinc reagent is ready for use.
[0035] Add 20mL DMAC to reaction flask 2, add 10g 3-bromophenylmethyl sulfone, 389mg Pd 2 (dba) 3 , 259mg of tris(o-methylphenyl)phosphorus, replaced by nitrogen three times, then heated to 70-80°C, added dropwise the organozinc reagent in reaction bottle 1, after the addition was completed, kept stirring for 1 hour and sent to LC-MS. After the reaction was complete, the reaction solution was cooled to r...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap