Igg1 fc mutants with ablated effector functions
A molecular and carrier technology, applied in the field of IgG1 Fc mutants whose effector function is eliminated, can solve the problems of reducing FcγR binding and identifying mutations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0180] 1. A recombinant IgG1 Fc-containing molecule comprising a CH2 domain in which the amino acid at position 265 is different from aspartic acid (D), position 297 The amino acid at position 329 is different from asparagine (N), the amino acid at position 329 is different from proline (P), where the numbering is indicated by the EU index in Kabat.
[0181] 2. The molecule of embodiment 1, wherein said molecule has, compared to an IgG1 Fc-containing molecule having a wild-type CH2 domain comprising D at position 265, N at position 297, and P at position 329, Reduced binding to C1q and to at least one Fcγ receptor (FcγR).
[0182] 3. The molecule of embodiment 1 or 2, wherein said molecule retains binding to FcRn.
[0183] 4. The molecule of any one of embodiments 2-3, wherein at least one FcyR is FcyRI, FcyRIIa, FcyRIIb, FcyRIIIa, and FcyRIIIb.
[0184] 5. The molecule of any one of embodiments 1-4, wherein
[0185] i. the amino acid at position 265 is alanine (A), asparag...
example
[0217] The following examples are provided to supplement the existing disclosure and provide a better understanding of the subject matter described herein. These examples should not be viewed as limiting the described subject matter. It should be understood that the examples and embodiments described herein are for illustrative purposes only, and that various modifications or changes therefrom will be apparent to those skilled in the art and are to be included therein and can be made without departing from this document. made without the scope of the invention.
example 1
[0218] Example 1. Expression and purification of Fc mutated antibodies
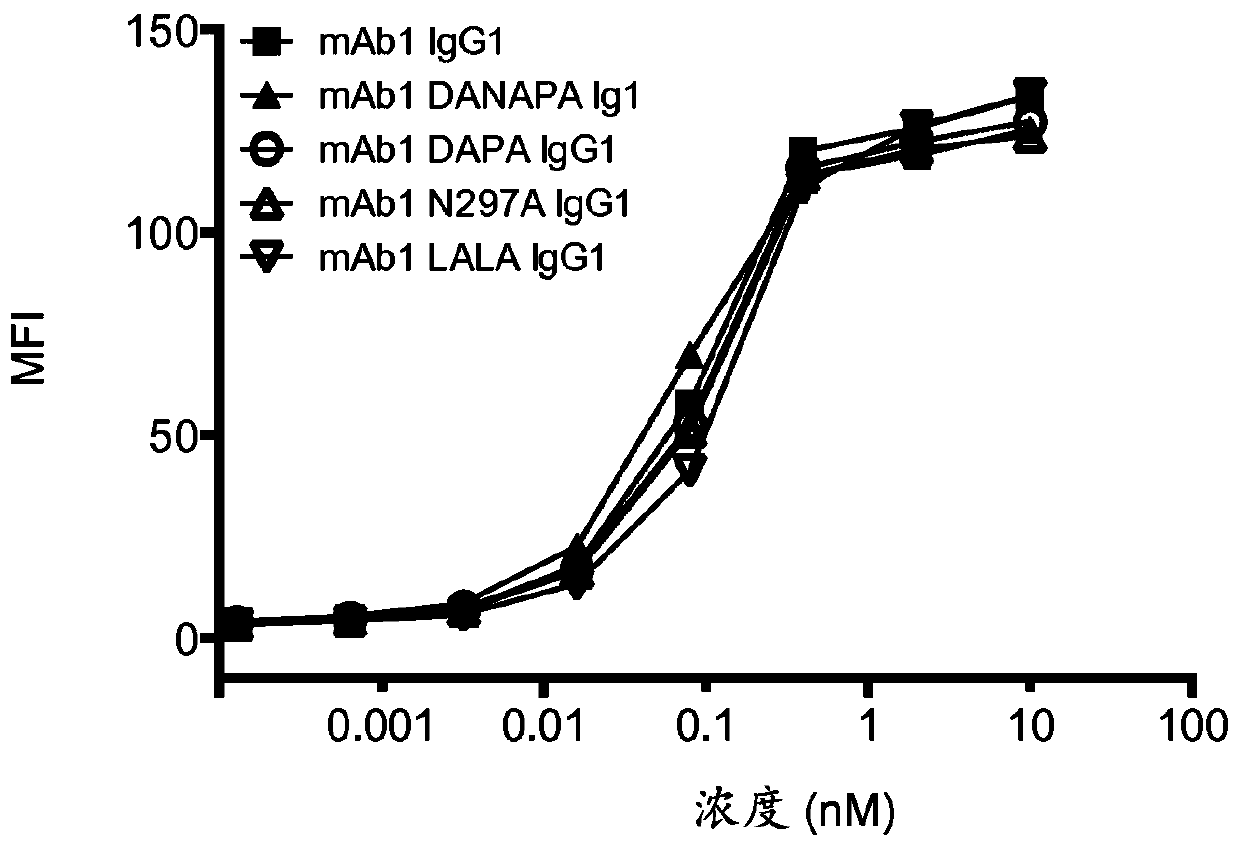
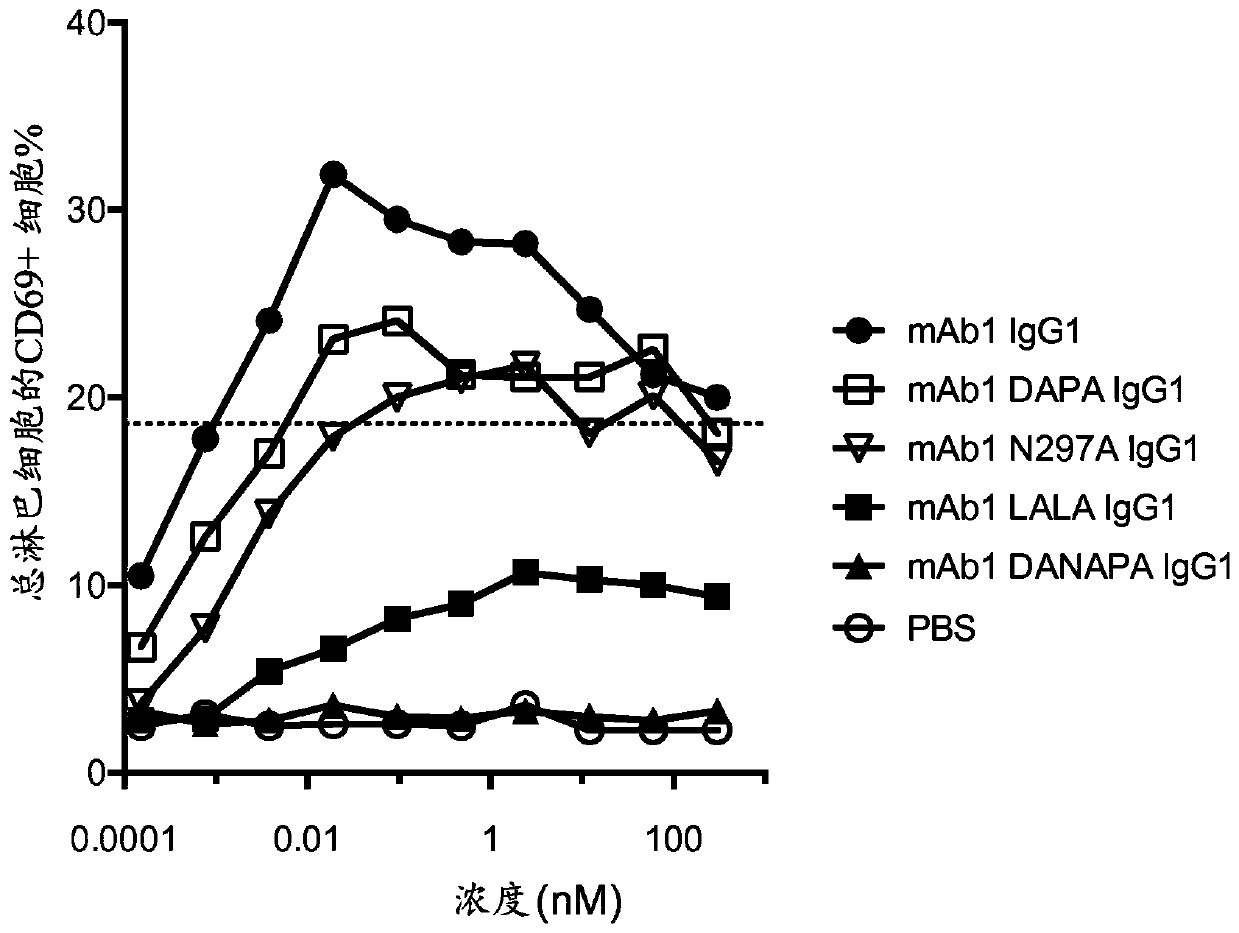
[0219] Several antibodies with different mutations in the CH2 domain were produced based on mAb1 (mAb1 is a human IgG1 antibody specific for human CD3). The mutations are:
[0220] -i) N297A,
[0221] -ii) D265 plus P329A (DAPA),
[0222] -iii) D265 plus N297A plus P329A (DANAPA), and
[0223] -iv) L234A plus L235A (LALA)
[0224] (EU numbering according to Kabat (Kabat, E.A. (1991). Sequences of proteins of immunological interest [ Protein sequences of immunological significance], Bethesda, MD: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, 1991).
[0225] For expression of antibodies, a leader sequence is usually present, which is excised and no longer present in the secreted product. An example of a leader sequence for expression in the examples described herein is provided in SEQ ID NO:42, and an example of a nucleotide sequence encoding the l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap