Preparation method of benzoylsulfamoyl benzamide and preparation intermediate
A kind of technology of benzoyl sulfamoyl benzamide and methoxy group, which is applied in the field of preparing intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
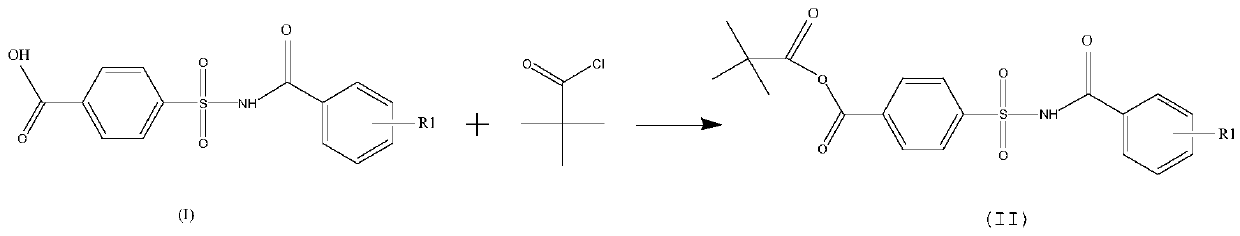
[0034] The new preparation method of the present invention comprises:
[0035] step one:
[0036]
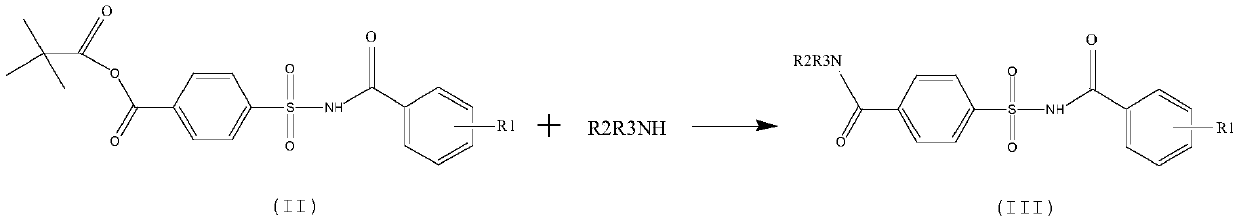
[0037] Step two:
[0038]
[0039] The reaction in the above step 1 can be carried out in solvents such as halogenated hydrocarbons, toluene, isopropyl acetate, ethyl acetate, tetrahydrofuran, dioxane, etc., and is particularly preferably carried out in dichloromethane. The reaction temperature is generally 15°C to 60°C, preferably 25°C to 35°C. The reaction time is 1 to 8 hours, preferably 1 to 6 hours. The molar ratio of the compound of formula (I) to pivaloyl chloride is preferably 1:1-1:2, more preferably 1:1-1:1.5, particularly preferably 1:1-1:1.1.
[0040] The reaction of step 1 is preferably carried out in the presence of a base. The base is preferably an organic base, including but not limited to triethylamine, diisopropylethylamine, pyridine, etc., preferably triethylamine. The amount of the base used is preferably 1 to 3 times the molar amount of the compou...
Embodiment 1
[0068] The preparation of embodiment 1N-(2-methoxy-benzoyl)-4-methyl-benzenesulfonamide
[0069]
[0070] Add 250 grams of isopropyl acetate to the three-necked flask, start stirring, add 110 grams of p-toluenesulfonamide, raise the temperature to 75°C to 80°C, then add 112.4 grams of o-methoxybenzoyl chloride dropwise, and the dropwise addition is completed in about 1 hour . After dropping, keep warm for 3 hours. Sampling was carried out for HPLC detection, and the remaining p-carboxybenzenesulfonamide was ≤0.5%, and the reaction was qualified. Cool down to 25°C, filter, rinse with 50 g of isopropyl acetate, and dry to obtain 187 g of the target product.
Embodiment 2
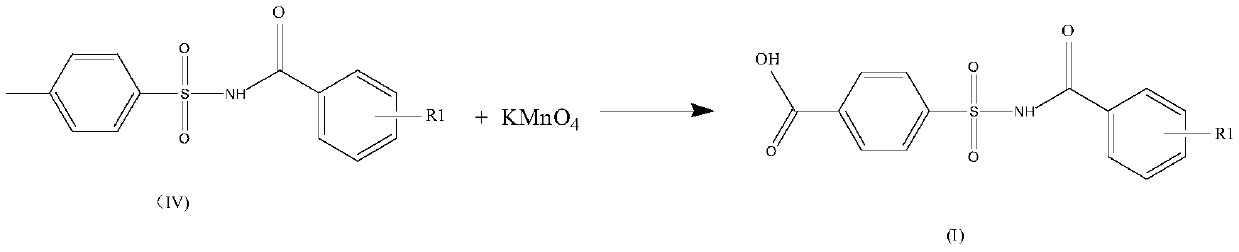
[0071] Example 2 Preparation of 4-(2-methoxy-benzoylsulfonyl)-benzoic acid
[0072]
[0073] First add 1500 grams of water in the three-necked flask, start stirring, add 187 grams of N-(2-methoxy-benzoyl)-4-methyl-benzenesulfonamide prepared in Example 1, and then add 49 grams sodium hydroxide. Control the temperature in a cold water bath to 25°C to 30°C, slowly add 194 grams of potassium permanganate, and dropwise complete in about 2 hours. After the addition was completed, the reaction was continued at 25°C to 30°C for 2h. Manganese dioxide was removed by filtration, and the filtrate was adjusted to pH 3-4 with hydrochloric acid, filtered, washed with water, and dried to obtain 191 grams of the product.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap