Medicine for treating thromboangiitis obliterans, preparation method and content determination method
A technology for thromboangiitis and vasculitis, which is applied in the field of traditional Chinese medicine, can solve the problem of no clear conclusion on the main etiology of thromboangiitis obliterans, and achieve the effect of restoring vasodilation, stabilizing the effect, and improving the inflammatory environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The drug (Xuebitong) for treating thromboangiitis obliterans is made into capsules with the following raw materials in grams: Astragalus 30g, Salvia miltiorrhiza 15g, Radix paeoniae Rubra 20g, Centipede 15g, Dilong 10g, Wood beetle 15g, and steroids Rattan 20g, Tangerine 15g, Uncaria 15g.
experiment example 1
[0038] Clinical Report on Xuebitong Capsules
[0039] 1. Observation on 50 Cases of Thromboangiitis Obliterans Treated by Xuebitong Capsules
[0040]
[0041] Conclusion: According to the accumulative clinical observation of 50 patients with thromboangiitis obliterans, the cure rate is 72%, the effective rate is 92%, the effective rate is 98%, and the ineffective rate is 2%. Significant effect.
[0042] 2. Diagnostic criteria for thromboangiitis obliterans:
[0043] 1. Male, aged 20-40. 2. Symptoms of chronic limb ischemia, such as numbness, fear of cold, intermittent claudication, nutritional disorders, etc., often involving the lower limbs, but rarely upper limbs. 3. 40-60% have a history or signs of migratory thrombotic superficial veins. 4. Various examinations have proved that the arterial occlusion and stenosis of the limbs are mostly in the popliteal artery and its distal arteries (often involving small and medium arteries). 5. History of smoking or exposure to co...
experiment example 2
[0056] Xuebitong preparation method, content determination method and curative effect verification
[0057] 2.1 Experimental materials
[0058] 2.1.1 Cells and animals
[0059] Cell line: human umbilical vein endothelial cells (HUVEC).
[0060] Animals: Clean grade SD rats, male, weighing 180-200g. Adaptive feeding for one week. All were purchased from Liaoning Changsheng Biotechnology Co., Ltd.
[0061] Experimental equipment, see Table 1.1
[0062] Table 1.1 Experimental Instruments
[0063]
[0064] 2.1.3 Experimental reagents, see Table 1.2
[0065] Table 1.2 Experimental Reagents
[0066]
[0067] 2.1.4 Medicinal materials
[0068] The standards of medicinal materials in this experiment are in accordance with the items of medicinal materials in the 2015 edition of the Chinese Pharmacopoeia.
[0069] Liquid phase conditions
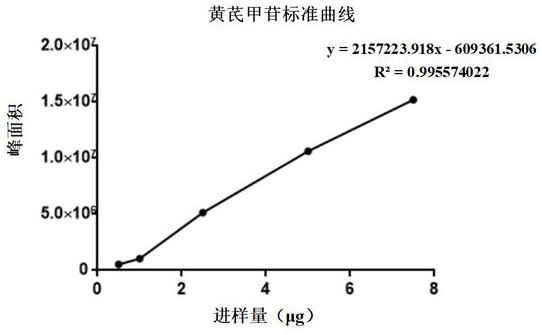
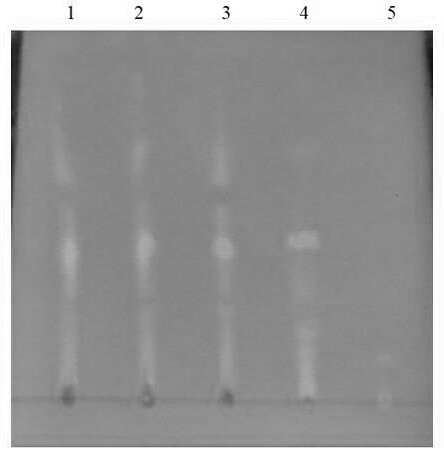
[0070]Astragaloside IV: InertSustain C18 column (5 μm, 4.6*250 mm) with octadecylsilane bonded silica gel as filler, acetonitrile-wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com